This part of an atom has a positive charge.
proton
The rows on the Periodic Table are called this.
periods
Hydrogen has this many valence electrons.
1
H2O has this many atoms.
3
List the Noble Numbers
2, 10, 18, 36, 54, 86
The part of the atom that has no charge (neutral).
neutron
The columns on the Periodic Table are called this.
groups
Neon has this many valence electrons.
0 or 8
C6H12O6 has this many atoms.
24
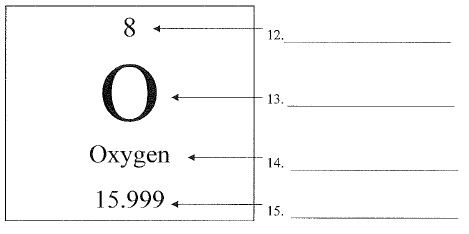 What is #12?
What is #12?
Protons and neutrons are found in this center part of the atom.
nucleus
The small number above (and sometimes to the upper left) is called this.
atomic number
Calcium has this many valence electrons.
2
3C2H3N5 has this many atoms.
30
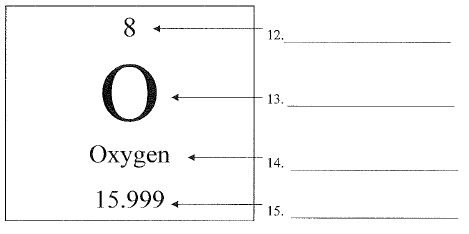 What is #15?
What is #15?
atomic mass or atomic weight
These negative parts of the atom are found in levels orbiting around the center.
electrons
The number at the bottom of the square below the letter is.
atomic mass
Titanium has this many valence electrons.
4
S4H5(F2Ti3)2 would have this many atoms.
19
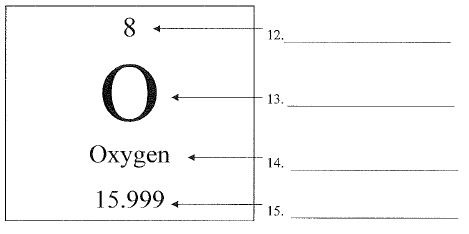 What is #14?
What is #14?
element name
This many electrons can fit in the first shell.
2
The letter (or letters) that is always a capital first and sometimes has a lower case letter after.
chemical symbol or elemental symbol
Bromine has this many valence electrons.
17
3K2(Mg3Na2)4 would have this many atoms.
66
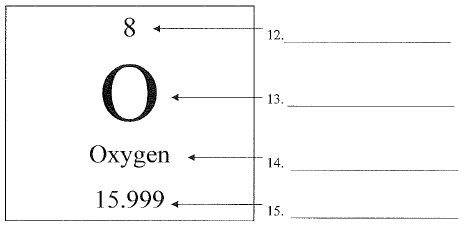 What is #13?
What is #13?
chemical symbol or element symbol
The sixth shell can hold this many electrons when full.
32
You find the number of neutrons in an atom by doing this.
atomic mass - atomic number
The group of elements that have full outer shells is called this.
Noble Gases
2CH4O3(Na4P)2 would have this many atoms.
36
 How many neutrons does Nitrogen have?
How many neutrons does Nitrogen have?
7