The number you use to find the number of protons in the nucleus.
What is the atomic number?
3 main states of matter
What are Solid, liquid, and Gas?
Ca
What is the element symbol for Calcium?
The chemical formula for Carbon Monoxide.
CO
Columns on the periodic table
Groups or families
Three main groups of elements are on the periodic table
What are metals, nonmetals, and metalloids?
Ar
TWhat is the element symbol for Argon?
The number of protons in the atom Beryllium
What is 4?
These are used to calculate the atomic mass of an atom
Protons & Neutrons
Elements that are the most abundant on the periodic table.
What is a Metal?
Stated that an atom's electrons are imbedded in it's center, like berries in a muffin.
Thomson
MgCl2
What is the chemical formula for Magnesium Chloride?
Protons and Neutrons
What subatomic particles are in the nucleus?
O₂
What is an oxygen molecule?
Name the scientist who created the periodic table of elements.
Mendeleev
Group 18 elements on the right hand side of the periodic table of elements.
What are the Noble Gases?
The 7 shown here is called this.
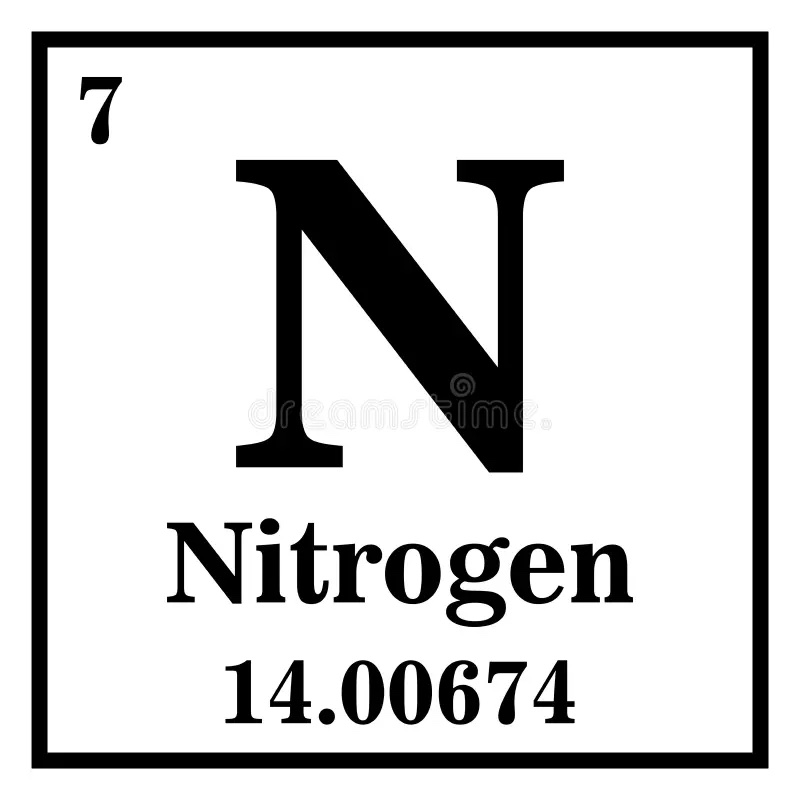
What is the atomic number of Nitrogen?
The electron
What subatomic particle is in the outside of the nucleus of an atom?
Alkali metals
What are the elements in Group 1 called ?
Name all 3 subatomic particles.
What are Protons, Neutrons, and Electrons
Ions are made in what way.
What is losing or gaining electrons?
Claimed that an atom resembles our solar system
Nagaoka
Ne
What is the symbol for the element neon?
The ___________ __________ tell us whether an element is a metal, nonmetal, or metalloid.
background color of an element's square
The sum of protons and neutrons in the nucleus of an atom is known as the _________
What is the mass number?
Said that electrons travel randomly around the nucleus
Rutherford
The 6 shown in this chemical formula.
C6H12O6
What is the number of carbon atoms and/or oxygen atoms?
Na
What is the element symbol for sodium?
26
How many protons are in Iron?
Where you find the elements with the largest atomic radius.
What is toward the bottom and left of the periodic table?
The elements in Group 1 or on the left of the periodic table
What are highly reactive elements or alkali metals?
Electrons travel in energy levels. The _________energy level holds a maximum of 2 electrons.
1st
First to show electrons move in specific energy levels.
Bohr
The Octet Rule describes this idea.
What is the number of electrons needed in the outer shell of an atom to be stable is 8?
A type of element with properties between metals and non-metals
What are metalloids?