Chemical Properties
Conservation of
Matter
Where are the electrons found in an atom?
In electron shells/clouds/rings on the outside of the nucleus.
How are elements arrange in the periodic table?
The elements are arrange in order of atomic number or number of protons.
What is the difference between a chemical and a physical change?
A physical change can be reversed and a chemical change cannot be reversed easily.
What is the simplest unit of matter?
An atom
According to the law of conservation of mass –
matter is not created or destroyed in a chemical reaction.
What are the three subatomic particle (small particles) that make up an atom?
Protons and Neutrons make up the nucleus of the atom and the electrons are found outside the nucleus.
What is the difference between a group and a period?
Groups form vertical columns and gives # of valence electrons. Periods are horizontal rows and gives # of energy shells.
What are three signs of a chemical change?
Color change, Formation of a gas, Temperature change, and Formation of a precipitate
Why do atoms bond or react with each other?
To become stable and have a full octet or 8 valence electrons.
The majority of an atom's volume comes from-
the electrons in motion around the nucleus
Draw the Lewis Structure of B
A capital B surrounded by three electrons.
What is the most reactive group of nonmetals?
Group 1 (The Halogens).
What are three physical properties of a metal?
Conductivity, Malleability, Magnetism, and Luster
Which of the following equations is correctly balanced?
A. N2 + 3H2 → 2NH3
B. H2 + O2 → 2H2O
C. 2Ag2O → Ag2 + O2
D. N2 + H2 → NH3
A. N2 + 3H2 → 2NH3
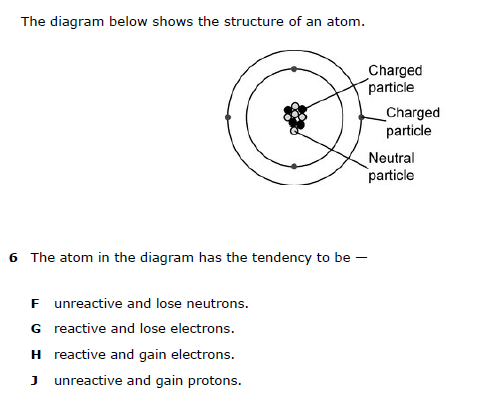
G. reactive and lose electrons
What subatomic particle do we use to identify an atom?
Protons
Why are the Noble Gases considered stable?
Each element in the Noble gases has a full outer electron shell or have at least 8 valence electrons (Helium has 2).
In which of the following examples has a chemical reaction occurred?
A After putting liquid batter into a hot pan, the batter solidifies and begins to turn brown.
B After turning on a blender filled with fruits, the fruit is chopped up and mixed together.
C After taking a tray filled with ice cubes out of the freezer, the cubes begin to melt.
D After adding a spoonful of sugar to a mug of hot coffee, the sugar dissolves completely.
A After putting liquid batter into a hot pan, the batter solidifies and begins to turn brown.
What are two facts that will always be true about the reactants and the products of a chemical reaction that takes place in a closed space?
The number of atoms in the reactants and the number of atoms in the products are the equal. The mass of the reactants and the mass of the products are equal.
What term best describes the change in temperature during a chemical reaction that causes the products to become cold (decrease in temperature)?
Endothermic Reaction
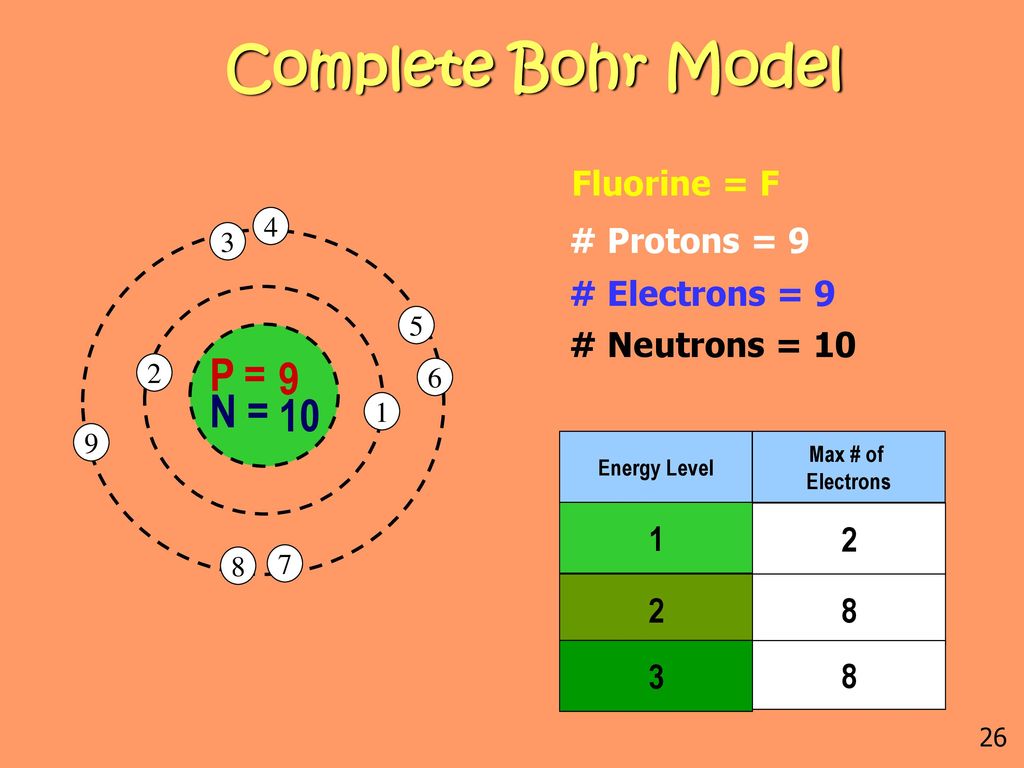
Draw the Bohr's Model for Fluorine.
List the following elements in order of their reactivity. Start with the most reactive and move to the least reactive. Ca , C, Br, K, He
K, Ca, Br, C, He
An experiment is performed in which a crystalline substance is added to a beaker filled with room temperature water. The following observations are made:
I. The crystals dissolve
II. A precipitate is formed.
III. The bottom of the beaker feels warm to the touch.
IV. Bubbles begin forming within the water.
All of the observations are evidence of a chemical change EXCEPT -
observation I.
An unbalanced equation is shown here. Below it is a diagram with four locations in the unbalanced equation labeled. The equation could be balanced by adding a number two (2) in which location?
B
What term best describes the change in temperature during a chemical reaction that causes the products to become hot (increase in temperature)?
Exothermic Reaction
