States of matter
What do carbon, hydrogen, and oxygen atoms have in common? (SC.8.P.8.7)
A. the same number of protons
B. the same number of electrons
C. neutrons located outside the nucleus
D. electrons located outside the nucleus
D. electrons located outside the nucleus
During a cooking activity, a student observes sugar heating on a pan until it melts and then starts to turn brown and smells burned. Which explanation best identifies what is happening to sugar and why? (SC.8.P.9.2)
A. Melting sugar is a chemical change because the sugar is heating and changing state from solid to a liquid.
B. Burning sugar is a chemical change because new substances, like caramelized sugar, are formed.
C. Melting sugar is a physical change because sugar dissolves.
D. Burning sugar is a physical change because it changes color
D. Burning sugar is a physical change because it changes color
If zinc (Zn) can conduct electricity, which of the following would also conduct electricity? (SC.8.P.8.6)
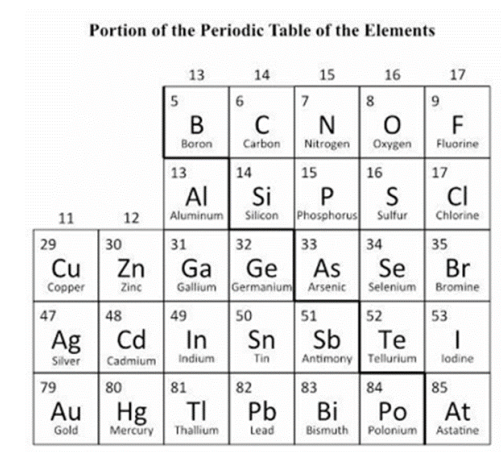
A. Bromine (Br)
B. Carbon (C)
C. Mercury (Hg)
D. Sulfur (S)
C. Mercury (Hg)
The graph below shows the mass in grams (g) of three liquids at the same temperature and with the same volume. Which conclusion about density can be made from the graph? (SC.8.P.8.4)
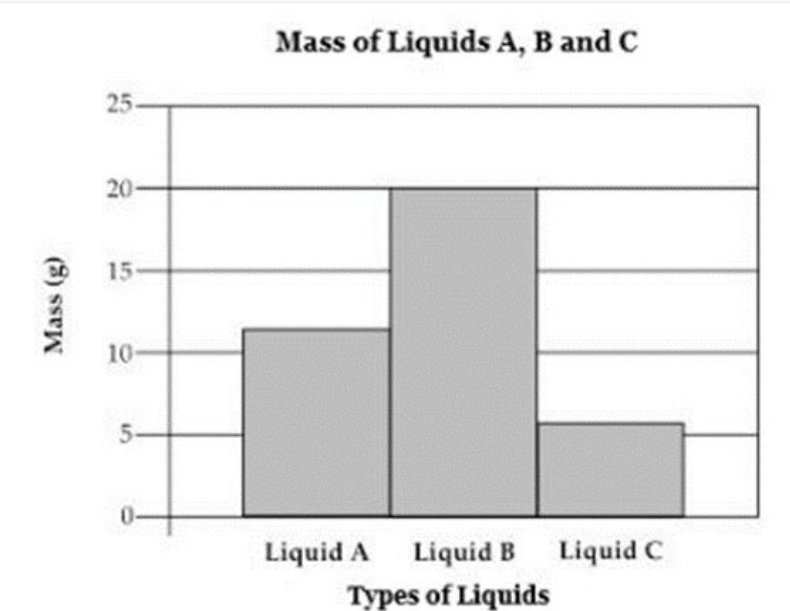
A. Density is not always related to mass and volume.
B. Different substances of equal volumes have the same density.
C. It is less difficult to measure the density of liquids than of solids.
D. Equal volumes of different substances may have different densities
D. Equal volumes of different substances may have different densities
Clean air is essential for the survival of organisms. Burning coal can produce sulfuric acid (H2SO4) that remains as mist in the atmosphere. This mist results in acid rain that can harm vegetation and eliminate aquatic life in various waterways. The following reaction occurs when coal burns:
Which term best defines H2SO4?
A. Atom B. Compound C. Element D. Isotope
B. Compound
Which of the following would substance X best represent? (SC.8.P.8.8)
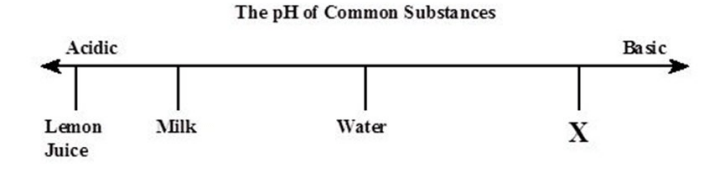
A. Soap
B. Vinegar
C. Lemonade
D. Orange juice
A. Soap
Which of the following best identifies how the items are grouped? (SC.8.P.8.9)
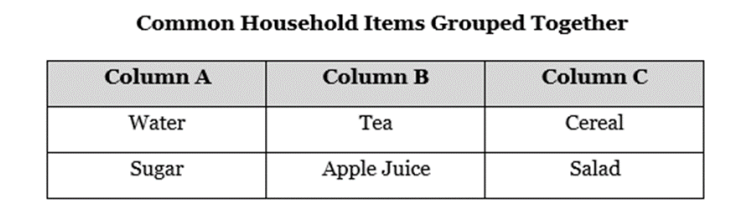
A. Column A are solutions, Column B are mixtures, and Column C are pure substances.
B. Column A are pure substances, Column B are mixtures, and Column C are solutions.
C. Column A are pure substances, Column B are solutions, and Column C are mixtures.
D. Column A are solutions, Column B are pure substances, and Column C are mixtures
C. Column A are pure substances, Column B are solutions, and Column C are mixtures.
Some acids and metals can chemically combine to form a salt and release a gas, as shown below. When testing the above reaction in a closed system, which of the following best describes the result of this chemical change? (SC.8.P.9.1)

A. The volume of the acid and the volume of the gas are constant during the chemical change.
B. The volume of the metal increases as the chemical change produces more gas
C. The mass of the acid and metal is equal to the mass of the gas and the salt.
D. The mass of the metal is greater than the mass of the salt.
C. The mass of the acid and metal is equal to the mass of the gas and the salt.
If a sample of silver is reduced to the smallest possible particle of pure silver, which statement would most accurately describe the result? (SC.8.P.8.7)
A. The sample would be reduced to an atom of silver.
B. The sample would be reduced to molecules of silver.
C. The sample would be reduced to protons and neutrons of silver.
D. The sample would be reduced to electrons and protons of silver
A. The sample would be reduced to an atom of silver.
A student dissolves salt in water to create saltwater solution. Afterward, the water is evaporated, leaving the salt behind. Which best explains whether this is a physical or chemical change? (SC.8.P.9.2)
A. It is a chemical change because salt disappears in water.
B. It is a chemical change because a new solution is formed.
C. It is a physical change because the salt retains its molecular structure and can be recovered.
D. It is a physical change because it involves water molecules needed for all physical changes.
C. It is a physical change because the salt retains its molecular structure and can be recovered.
Robyn and Sam were examining the periodic table and discussing how elements were grouped based on similar properties. Which best describes a group of elements in the periodic table with similar properties? (SC.8.P.8.6)
A. Elements that are listed in the same row.
B. Elements that are listed in the same column.
C. Elements that are listed near each other in any direction.
D. Elements that are listed on opposite sides of columns or rows
B. Elements that are listed in the same column.
Density is defined as the amount of matter contained in a specific volume. The formula for density is given below. If a solid metal cube has a density of 4.0 g/cm3 and a mass of 12.0 grams, what is its volume? (SC.8.P.8.3)
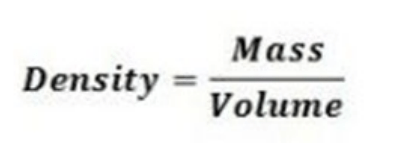
A. 0.33 cm3 B. 3.0 cm3 C. 16.0 cm3 D. 36.0 cm3
B. 3.0 cm3
Water (H2O), rust (Fe2O3), and table salt (NaCl) are common substances found on Earth. What do water, rust, and table salt have in common? (SC.8.P.8.5)
A. Water, rust, and table salt are atoms.
B. Water, rust, and table salt are mixtures.
C. Water, rust, and table salt are elements.
D. Water, rust, and table salt are compounds
D. Water, rust, and table salt are compounds
Litmus is a specially treated paper that changes color when it comes in contact with acids and bases. For example, litmus will remain blue in bases but turns red in acids. Which of the following will most likely turn litmus red? (SC.8.P.8.8)
A. Baking soda
B. Orange juice
C. Soap
D. Spring water
B. Orange juice
She labels a test tube for each substance, 1 through 3, and places each substance into them. In Test Tube 4 she pours equal amounts of both Substance 2 and Substance 3. A diagram of her setup is shown below.
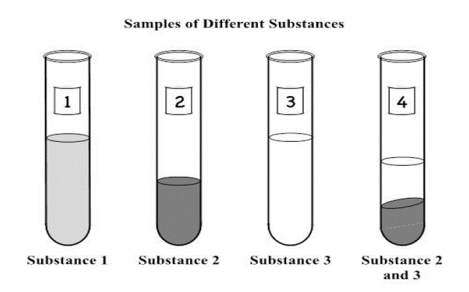
Dana is trying to determine if an unknown sample is a pure substance or not. The sample has a cloudy white color, and it seems evenly mixed throughout. The sample is a thick liquid. Dana tried separating the sample with a magnet with no effect. When Dana filtered the sample, larger granules of the sample were left behind in the filter. What evidence allowed Dana to conclude that the sample is not a pure substance? (SC.8.P.8.9)
A. It is cloudy white.
B. It is a thick liquid.
C. Dana tried separating the sample with a magnet with no effect.
D. Dana filtered the sample and larger granules of the sample were left behind.
D. Dana filtered the sample and larger granules of the sample were left behind.
Susan is investigating physical changes. To do this, she places some ice into a large bowl and seals it with a lid. She leaves the bowl on the counter for several hours until all of the ice has melted. Using a balance, Susan determines that the mass of both the water and the ice are equal. Why is the mass of the ice and the water the same? (SC.8.P.9.1)
A. The larger ice atoms change mass into heat as they melt into smaller water atoms.
B. The number of atoms in the ice is the same as the number of atoms in the water.
C. Physical changes can only increase the density of a substance.
D. Chemical changes can only decrease the mass of a substance
B. The number of atoms in the ice is the same as the number of atoms in the water.
Atoms are very small structures, however they are made up of even smaller particles. One of these particles is called the electron. Which of the following best describes an electron? (SC.8.P.8.7)
A. It has no charge and about the same mass as a proton.
B. It has a negative charge and much less mass than a proton.
C. It has a positive charge and much more mass than a neutron.
D. It has a negative charge and about the same mass as a neutron.
B. It has a negative charge and much less mass than a proton.
Erin puts one antacid tablet in a glass of cool water and records how long it takes to stop fizzing. She repeats the same procedure with a glass of hot water. Erin notices that the hot water finishes fizzing quicker than the cold water. Which of the following is the best conclusion using Erin’s results? (SC.8.P.9.3)
A. Hot temperatures can increase the speed of a chemical change.
B. Chemical reactions are not affected by temperature.
C. Cold temperatures can stop a chemical change.
D. Chemical reactions are usually very fast
A. Hot temperatures can increase the speed of a chemical change.
Based on how the periodic table is arranged, what can best be inferred about the atomic number and atomic mass of Element X? (SC.8.P.8.6)
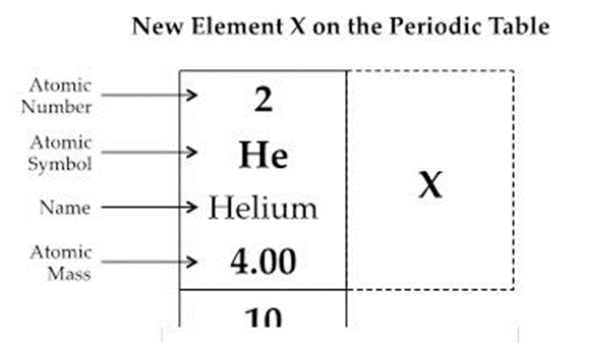
A. The atomic number and the atomic mass will both be higher than helium.
B. The atomic number will be higher and the atomic mass will be lower than helium.
C. Only the atomic number will remain the same as helium, the atomic mass will be higher.
D. Only the atomic number will be higher that helium, the atomic mass will remain the same.
A. The atomic number and the atomic mass will both be higher than helium.
Alex was completing an experiment with three different metals including Iron, Nickel and Zinc. For each of the three metals, she found the mass in grams and the volume of each sample in cubic centimeters. Using the formula, (Density = mass / volume), and the data table below, assist Alex with placing the metals in order from most dense to least dense. (SC.8.P.8.3)
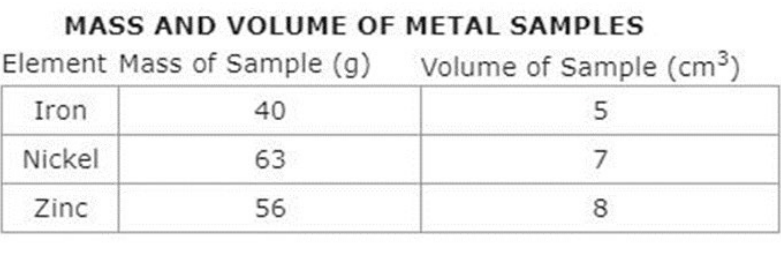
A. nickel, zinc, iron
B. nickel, iron, zinc
C. zinc, iron, nickel
D. zinc, nickel, iron
B. nickel, iron, zinc
Even though it is not found on the periodic table of the elements, water is one of several substances that are essential for life on Earth. Which of the following best explains why water is NOT grouped with the elements? (SC.8.P.8.5)
A. Water is a liquid.
B. Water is a mixture.
C. Water is a compound.
D. Water is a pure substance
C. Water is a compound.
Which sample from the table is the most acidic? (SC.8.P.8.8)
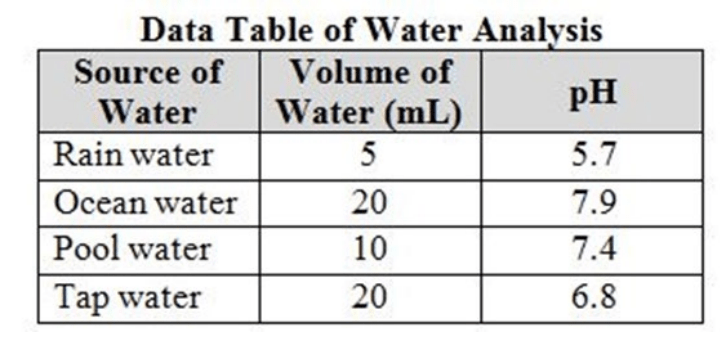
A. Tap water
B. Pool water
C. Rain water
D. Ocean water
C. Rain water
She labels a test tube for each substance, 1 through 3, and places each substance into them. In Test Tube 4 she pours equal amounts of both Substance 2 and Substance 3. A diagram of her setup is shown below. Which sample contains a heterogeneous mixture? (SC.8.P.8.9)
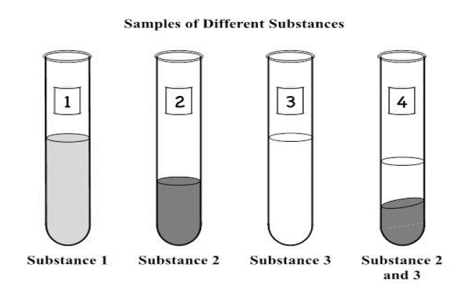
A. Test Tube 1 B. Test Tube 2 C. Test Tube 3 D. Test Tube 4
D. Test Tube 4
Melissa adds 10g of baking soda to 100g of vinegar. The mixture begins to bubble. When the bubbling stops, Melissa finds the mass of the resulting mixture. She determines its mass is 105g. Which reasoning describes why the mass has changed?
A A gas formed and left the mixture.
B Vinegar evaporated during the experiment.
C Mixtures are always less massive than their parts.
D Mass was destroyed when vinegar reacted with baking soda.
A A gas formed and left the mixture.
What structure in the model represents the nucleus? (SC.8.P.8.7)
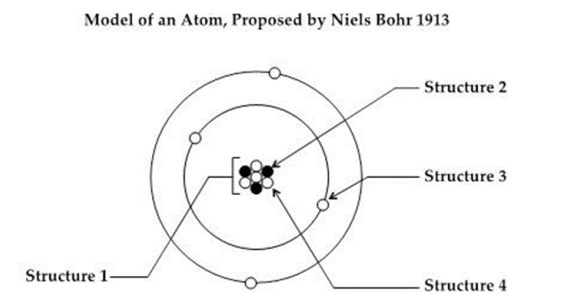
A. Structure 1
B. Structure 2
C. Structure 3
D. Structure 4
A. Structure 1
A student was working with a metal block and noticed that it acted like a magnet. The metal block fell and broke into several smaller pieces. Which of the following best identifies the properties of the smaller pieces? (SC.8.P.8.4)
A. The smaller pieces will have different melting points
B. Some pieces will have different densities.
C. All of the pieces will be soluble in water.
D. All of the pieces will be magnetic
D. All of the pieces will be magnetic
Silicon is a crucial component in microelectronics and computer chips and is known as a metalloid. Which statement describes the properties of metalloids? (SC.8.P.8.6)
A. Metalloids are much shinier than nonmetals and metals.
B. Metalloids can have properties of metals and nonmetals.
C. Metalloids are more reactive than metals and nonmetals.
D. Metalloids conduct less heat and electricity than nonmetals and metals
B. Metalloids can have properties of metals and nonmetals.
Four different liquids (substances w, x, y and z) are stacked one on top of another in the test tube above. Using the graduated cylinder and the data table above, determine which layer represents substance W. (SC.8.P.8.3)
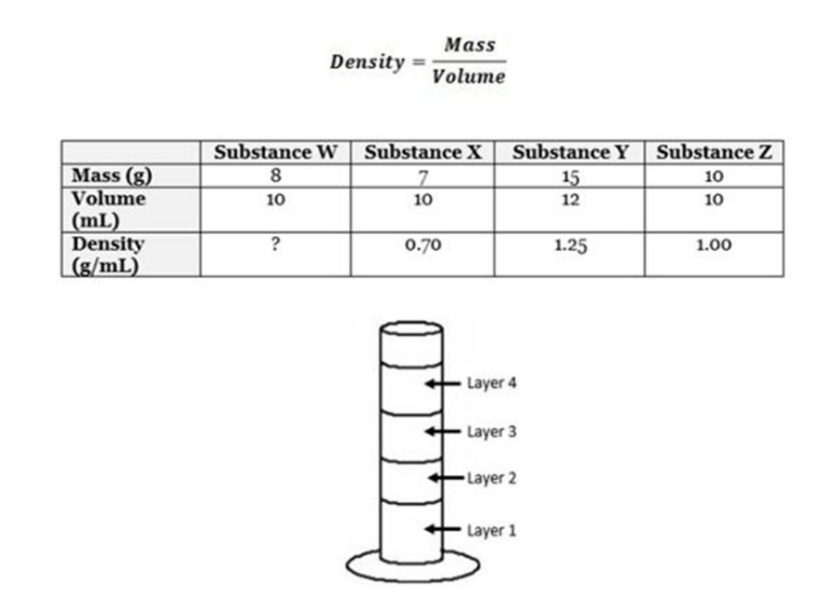
A. Layer 1 B. Layer 2 C. Layer 3 D. Layer 4
C. Layer 3
Under the right conditions, sodium (Na) and chlorine (Cl) can chemically combine to form Sodium Chloride. Which of the following best explains how sodium and chlorine are able to create sodium chloride? (SC.8.P.8.5)
A. Sodium and chlorine exchange neutrons between each other when they combine to form sodium chloride.
B. Electrons are transferred between sodium and chlorine when they combine to form sodium chloride.
C. Extra protons are created when both sodium and chlorine combine to form sodium chloride.
D. The nucleus of both sodium and chlorine combine together to form sodium chloride
B. Electrons are transferred between sodium and chlorine when they combine to form sodium chloride.
Anthony was conducting an experiment to determine what would happen to the pH of a liquid when he combined one substance with another substance. To conduct his research, Anthony started off with a small beaker filled with 10 mL of acetic acid. Using a pH probe, Anthony found the initial pH of the acetic acid was 4.0, which he recorded on the graph below. Anthony slowly added 1 mL of a “mystery solution” into the beaker at a time, until 17 mL were added. Every time Anthony added 1 mL of the mystery solution, he measured the pH of the new substance. Anthony recorded the pH and placed his measurements on the graph below.
Change in pH when adding the "Mystery Solution" to 10 mL of Acetic Acid
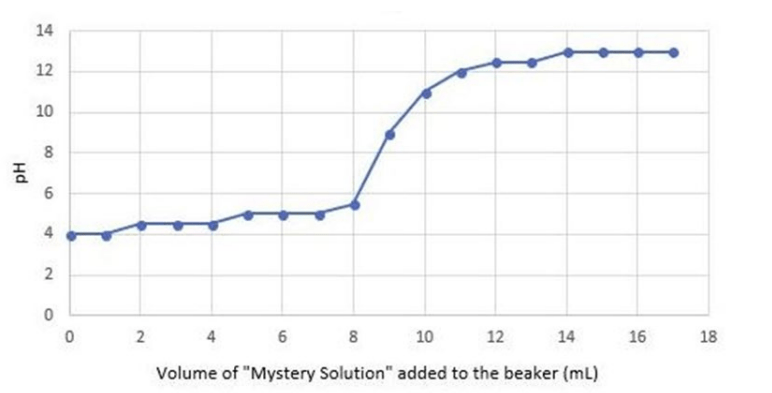
Using the information about the experiment and graph above, what we can conclude of the pH of the mystery solution? (SC.8.P.8.8)
A. it is neutral B. it is a weak base C. it is a strong base D. it is a strong acid
C. it is a strong base
The Earth is made up of different systems such as the geosphere, hydrosphere, and atmosphere, to name a few. Which statement best describes the air in Earth's atmosphere? (SC.8.P.8.9)
A. a pure substance made of one kind of gas
B. a solution of gases that are physically mixed
C. a compound made of two or more kinds of gases
D. a mixture of two gases that are chemically combine
B. a solution of gases that are physically mixed
Amber was researching the properties of water and found that it can exist in three different phases, including solid, liquid and gas. During her research, she found that particles have different amounts of kinetic energy based on the state of matter they are in. She wanted to share this information with her friend by listing the states of matter in sequence from most to least kinetic energy. Which list below shows the particles in the different phases of matter, in order from most kinetic energy to least kinetic energy? (SC.8.P.8.1)
A. solid, liquid, gas
B. gas, solid, liquid
C. solid, gas, liquid
D. gas, liquid, solid
D. gas, liquid, solid
Audra is looking at a diagram of an atom. Which particle(s) does she see inside the nucleus? (SC.8.P.8.7)
A. electrons only
B. neutrons only
C. electrons and protons
D. protons and neutrons
D. protons and neutrons
Matthew has six samples of different materials all of the same mass. He sorts them into two groups using one physical property. Which physical property did Matthew most likely use to sort the samples into two groups? (SC.8.P.8.4)
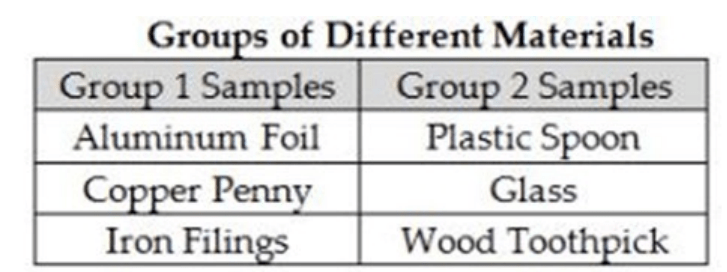
A. Magnetism B. Melting point C. Solubility in water D. Electrical conductivity
D. Electrical conductivity
Gracie wanted to classify an element, so she observed and tested several of its properties. She made the following table using her results.
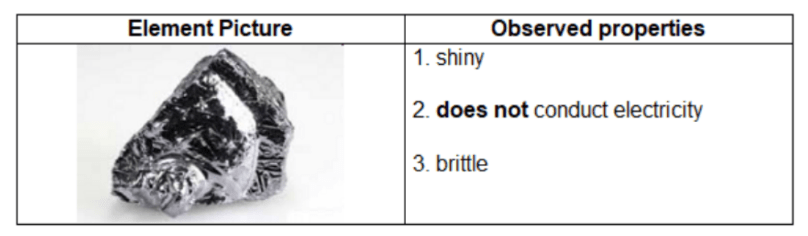
Based on these observations, how should Gracie classify her element?
A metal
B metalloid
C nonmetal
D compound
B metalloid
The equation for density is shown below.
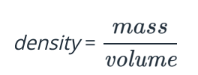
The density of gold is 19.3 g/cm3 . The density of iron is 7.9 g/cm3 . A sample of silver has a volume of 4.0cm3 and a mass of 42 g. How does silver compare with gold and iron in density?
A Silver is denser than either gold or iron.
B Silver is less dense than either gold or iron.
C Silver is denser than gold and less dense than iron.
D Silver is denser than iron and less dense than gold.
D Silver is denser than iron and less dense than gold.
Sodium (Na) is a silvery, soft metal that reacts explosively with water. Chlorine (Cl) is a greenish yellow gas at room temperature. When sodium bonds with chlorine, it creates a compound called table salt. Which of the following statements is true of compounds?
A Compounds will have the same properties as individual atoms that bonded together to make compounds.
B Compounds are mixtures of two or more different substances that are NOT chemically bonded.
C Compounds are new substances with different properties from the atoms that bonded to make them.
D Compounds are only created when one substance dissolves into another.
C Compounds are new substances with different properties from the atoms that bonded to make them.
When an acid and a base combine, they undergo a chemical change and produce a salt. Which of the following is an example of a salt produced from an acid and a base? (SC.8.P.8.8)
A. Water (H2O)
B. Carbon dioxide (CO2)
C. Sodium metal (Na)
D. Magnesium sulfate (MgSO4)
D. Magnesium sulfate (MgSO4)
When studying properties of matter you may come across there are heterogeneous mixtures and homogeneous mixtures (solutions). Which statement shows how homogeneous mixtures (solutions) can be separated into individual components? (SC.8.P.8.9)
A. Water can be separated into hydrogen and oxygen atoms using electricity.
B. The iron filings in a pile of iron filings and salt can be separated with a magnet.
C. Evaporation of water from saltwater will leave behind the salt that once was dissolved.
D. Salad dressing separates into individual layers of oil and vinegar when left to settle.
C. Evaporation of water from saltwater will leave behind the salt that once was dissolved.
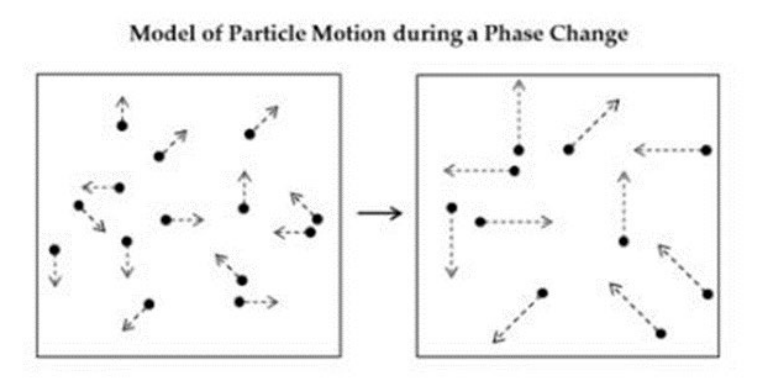
Which phase change is most likely modeled in the diagram? (SC.8.P.8.1)
A. A gas to a solid
B. A gas to a liquid
C. A liquid to a gas
D. A liquid to a solid
C. A liquid to a gas