Name 3 states of matter
Solid, liquid, gas
Mass is never ____________ or __________, it just changes ________________.
Mass is never created or destroyed, it just changes form.
Give three examples of mixtures.
Pizza, salad, smoothies
Give three examples of a chemical change
Fire, acid bubbling, baking cookies
Physical Change, Chemical Change, or Both?
Both:
The wick burning is a chemical change, but the wax melting is a physical change.
Why does an ice cube melt at room temp?
Temperature of the ice increases
Explain the difference between weight and mass on the moon.
If you go to the moon, your weight will change because the pull of gravity also changes. Your mass will remain the same.
What is it called when one substance completely dissolves in another substance?
A solution
Describe the difference between a physical change and a chemical change.
In a physical change, the chemical properties of a substance do not change and it can be returned to its original state. In a chemical change, the chemical properties of a substance changes and it can not be reversed.
 Describe the type of matter seen in this picture:
Describe the type of matter seen in this picture:
This matter is a solid form of water.
At an object’s boiling point, a ___________ changes to a ____________
At an object’s freezing point, a ___________ changes to a ____________
Solid, Liquid, or Gas
At an object’s boiling point, a liquid changes to a gas.
At an object’s freezing point, a liquid changes to a solid.
Your science teacher crushes a can of soda.
What has changed about the can?
Its shape
Its mass
Its weight
Its shape and weight
1. Its shape
Why is a solution like saltwater considered a physical change and not a chemical change?
The chemical properties of the salt and the water do not change when they are mixed together. The solution can also be separated.
Name three clues that point to the fact that a chemical change has occurred.
Change in color
Change in texture
Bubbles and gas are released
Describe the two types of matter seen in this picture:

The balloons are a solid form of matter, and the helium inside is a gas.
Explain how matter moves from a solid, to a liquid, to a gas.
In order to move from a solid to a liquid, the temperature of matter needs to increase.
To move from a liquid to a gas, the temperature needs to increase even more. This causes the particles to move faster.
Name two ways to measure volume.
Length x Width x Height
Water Displacement
Describe how to separate a solution of Kool-Aid and water.
You can boil the solution. When the water evaporates, the Kool-Aid crystals will be left behind.
Describe how you can cause both a physical change and a chemical change to this sandwich: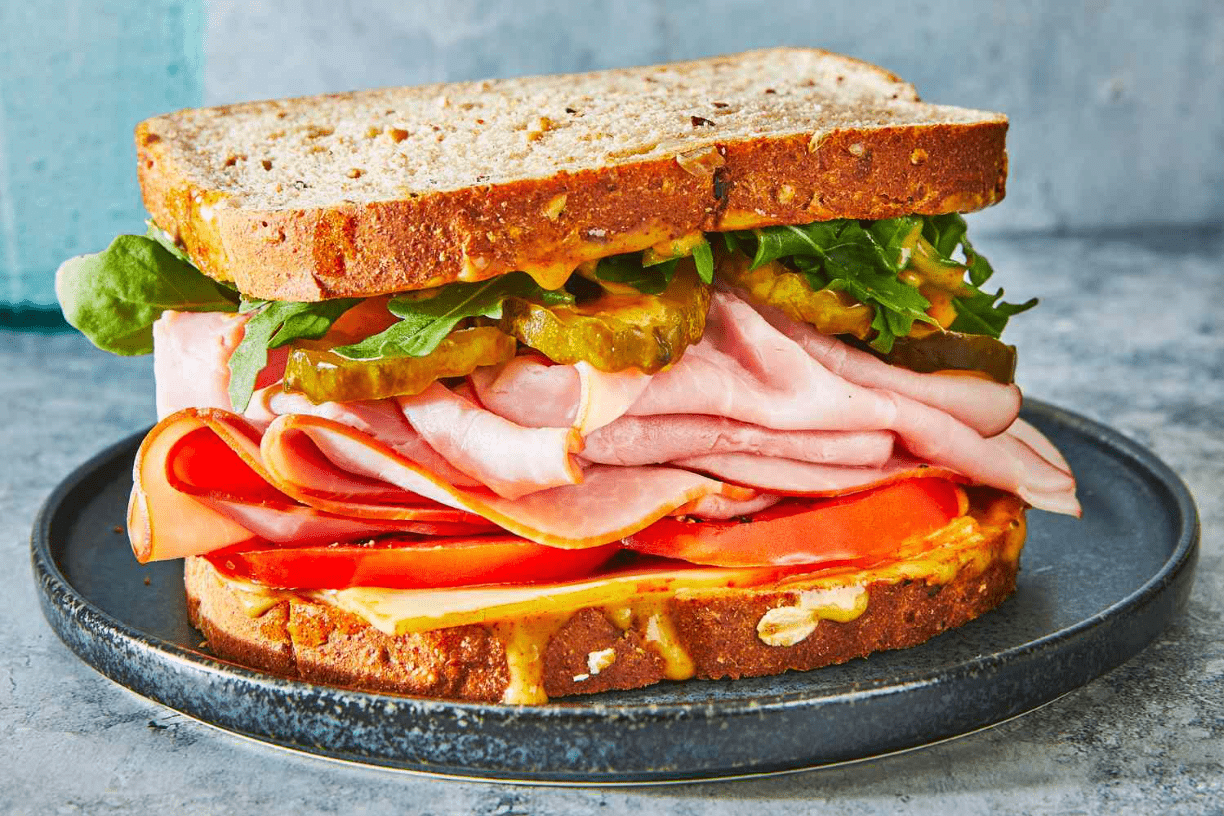
Physical Change: you can remove parts of the sandwich that you do not like to eat.
Chemical Change: you can toast the sandwich
Describe a physical change in this picture.
The physical change is the graham crackers mixed with the marshmallow.
Explain how condensation occurs
When the temperature drops (like on a glass with ice), gas particles in the air slow down and turn back into a liquid.
Using your knowledge of density, explain the following picture:
In this picture, the lamp oil has fewer particles than the rubbing alcohol; therefore, it floats because it has less density. The honey is the most dense liquid; therefore, it is on the bottom. 
Describe the steps that you would take to separate a mixture of:
Water
Salt
Iron filings
Rocks
Sand
Pick out the rocks with your hand
Use a magnet to take out the iron filings
Use a filter to remove the sand
Boil the water in order to turn it into a gas
The salt will be left behind
Explain why dying your hair is a chemical change and not a physical change.
When you dye your hair, you are using chemicals to alter the color of your hair. The only way to reverse it is to allow your hair to grow back.
Describe a chemical change in this picture and the sign that shows it is a chemical change.
The chemical change in this picture is the burnt marshmallow. That is a new type of matter.