How many valence electrons does H (hydrogen) have?
What is: 1
The metric unit of Mass
What is: Grams/Kilograms/Milligrams
Elements are arranged in groups according to what?
What is their number of valence electrons and they have similar properties.
Wich law is directly proportional?
What is: Charles Law
What does alpha decay do to the atomic # of an atom?
What is: It decreases it by 2.
What period is Na (sodium) in?
What is: 3
Name the property.
Describes a substance's ability to explode when exposed to sulfur.
What is: A chemical property
Define Viscosity.
What is: A liquids resistance to flow.
Name one trait of a halogen?
What is: Most reactive non-metals/commonly forms salts.
What is the charge of an ion of Si (Silicon)?
What is: -4,+4.
AIRPLANE!!!
If you had to get to California but ould only go over 1 state which one would you use?
What is: New Mexico
What is an orange? A compound, element, or mixture.
What is: A mixture
What is the freezing point of a liquid that has a melting point of 36 degrees celsius?
What is: 36 degrees celsius.
What is the most common use of radiation?
What is: In cancer treatment as they are harmful to live cells and 1,735,350 get cancer per year.
Name the 3 major types of decay.
What is: Alpha decay, Beta Decay, Gamma Decay.
What is the most conductive element?
What is: Silver and Gold
What is the appropriate kind of graph for the table?
Year Made: Money Made:
2010 1,025.1 Million
2014 758.6 Million
2016 967.3 Million
2017 1,123.7 Million
What is: Bar Graph
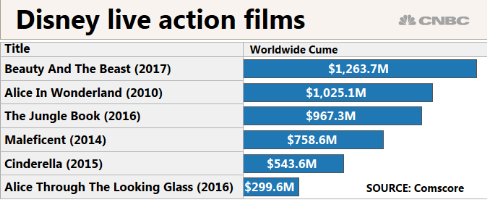
There are 3 main types of elements what are they and where are they placed?
NO LOOKING ON THE TABLE
What is: Metals on the right, Non-metals on the left, and Metalloids between metals and metalloids.
If the pressure in increases what happens to any substances properties?
Lower Volume Higher boiling point.
What makes beta decay more dangerous than alpha decay?
It is smaller so it can seep through imperfections in materials.
What is the world's longest living animal?
What is: The Bowhead Whale with an avg. lifespan of 300 years
If an irregular object is placed in a graduated cylinder with 100mL of water. The cylinder now reads 150mL. If the mass of the object is 50 grams, what is the density?
HINT: D=M/V
2.
What is: 1 g/mL
What is the age of a fossil that has 150 parents and 450 daughters, with the parents having a half-life of 1 year?
2. What is the age of a wool blanket that has 350 parents and 350 daughters with a half-life of 1 year?
What is the age of a nylon blanket with 300 parents and 300 daughters with a half-life of 3 millenniums?
2 Years
600/2= 300/2= 150
1*2=2
2. 700/2=350 350*1= 350
3. What is: There is no decay dating on artificial materials.
What is the most commonly found noble gas?
2. What is the most commonly found element
What is: Ar (argon)
2. What is: Carbon-14
How many alpha and beta decays do you need to get from Be-9 to Li-5?
2. How many alpha and beta decays do you need to get from Na-24 to Na-20
What is: One alpha and one beta.
2. What is: One alpha and two beta