Theory
Theory
Table
of Matter
of Matter
Conservation
How many protons are in the nucleus of a gold atom?

79 Protons
The vertical columns of the periodic table are called...
Groups or families.
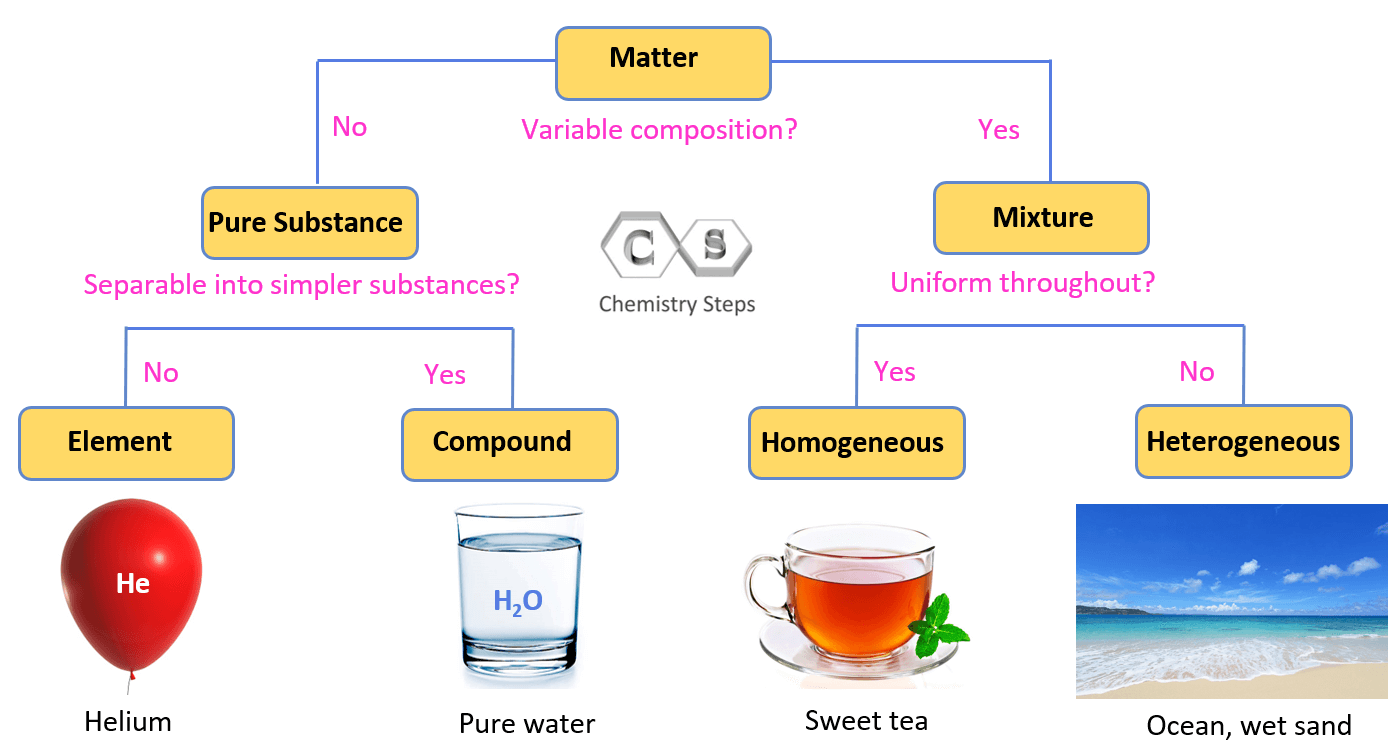
Define pure substances.
A pure substance is an element or compound and cannot be separated by physical means.

Which image depicts a solid? How do you know?
The first image is a solid because it has a definite shape and volume. The particles are tightly packed and vibrate in place.
What type of change is tearing a piece of paper: chemical or physical?
Physical change
What does the Law of Conservation of Matter state?
Matter cannot be created or destroyed, only changed.
How many neutrons are in the nucleus of a gold atom?

There are 118 neutrons in the nucleus of a gold atom because the atomic mass is 197 and the atomic number is 79. 197-79= 118
The vertical rows of a periodic table are called... and the atomic numbers ___ as you read from left to right.
The vertical rows of a periodic table are called PERIODS and the atomic numbers INCREASE as you read from left to right.
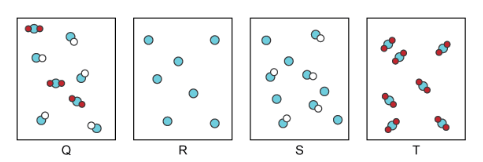
Which of the images below depicts pure substances?
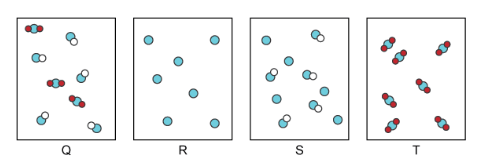
Image R is an element which is a pure substance, you only see single atoms of the same element.
Image T is a compound, which is 2 or more atoms chemically combined. All the compounds in image T are the same.
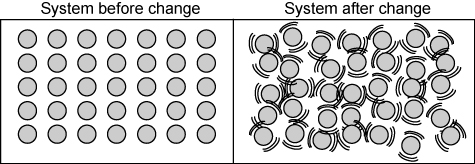
What happens to the particle motion of a substance when thermal head is added?
When thermal energy is added to a substance, particle motion increases. If enough thermal energy is added, there may be a change of states from solid to liquid or liquid to gas.
Which of the following is a chemical change: melting ice, boiling water, or rusting iron?
Rusting iron
In a closed system, if you start with 100 grams of reactants, how much product should you end with?
100 grams
What is the location AND charge of the subatomic particles of an atom?

Oxygen and sulfur are in the same group or family on the periodic table, what can you infer about them?
They have similar properties.

Is trail mix a homogenous or heterogenous mixture? Why?

Trail mix is a heterogeneous mixture because its components, such as candy, seeds, and dried fruits, are not uniformly distributed and remain distinct and visible
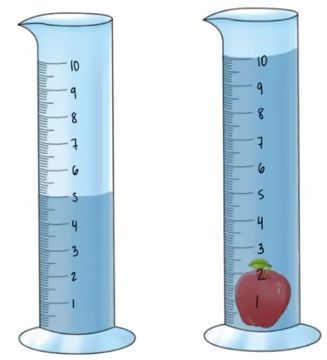
What is the density of an apple toy if the mass is 20 grams and the volume is pictured below.
Density = Mass / Volume
Mass= 20g
Volume= 5 cm3
20/5= 4g/cm3
Name one clue or evidence of a chemical change has occurred.

Color change, gas production/bubbles, temperature change, formation of a precipitate, or change in odor
Why must chemical equations be balanced according to the Law of Conservation of Matter?
Because the same number of atoms of each element must be on both sides of the equation.
What is the charge of the nucleus of an atom?
The nucleus contains protons which are positive and neutrons which have no charge so... the nucleus of an atom is positive.
What is the name, chemical symbol, and atomic number of the element in period 5, group 11 of the periodic table?
The element in period 5, group 11 of the periodic table is Silver (Ag), with the atomic number 47.

What type of mixture is sand and water? Why?

Sand is a heterogeneous mixture because it is composed of various particles of different sizes, shapes, and mineral identities that are not uniformly distributed throughout the substance. Its composition and appearance vary from one part to another, which is characteristic of heterogeneous mixtures.
Which metal has the highest density?
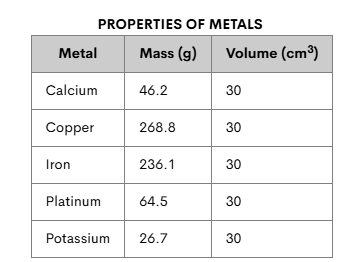
Copper has the highest density because it has the most mass per given volume.
268.8g / 30 cm3= 8.96 g/cm3
Is cooking an egg a chemical or physical change, and why?
A chemical change because new substances are formed and the change cannot be reversed.
During a reaction, 12 g of hydrogen reacts with 88 g of oxygen. How many grams of water are produced?
100 grams, because mass is conserved.
What do the atomic number and atomic mass tell us about the subatomic particles of an atom?
The atomic number tells us the number of protons and electrons of an element's atom. The atomic mass tells us the combined mass of protons and neutrons.
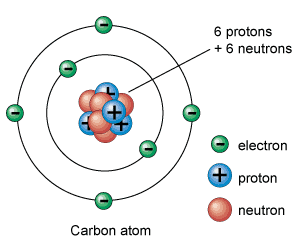
Describe or draw an atomic model of Carbon using the periodic table.
The atomic number of Carbon is 6, meaning that it has 6 protons in the nucleus and 6 electrons in the electron cloud. The atomic mass of Carbon is 12, and 12-6= 6 so there are 6 neutrons also in the nucleus of a Carbon atom.
Name one example of a homogenous mixture and one example of a heterogenous mixture. Explain your answer.
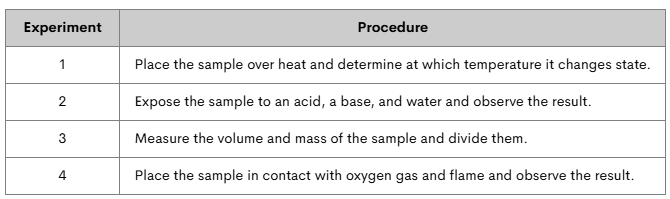
Marcus has an unknown substance. He conducts a series of experiments to determine some of its properties. Which experiments tested for physical properties? Which tested for chemical properties?
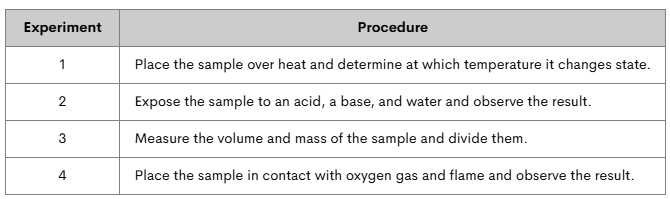
Experiment 1 tests boiling point= physical property.
Experiment 2 tests reactivity= chemical property.
Experiment 3 tests density= physical property.
Experiment 4 tests combustibility= chemical property.
Burning a candle involves both a physical and a chemical change. Explain how it's both.

The melting wax is a physical change because it changes state from solid to liquid without changing its chemical composition, and the burning wick is a chemical change because it produces new substances like carbon dioxide, water vapor, and heat.
In a chemical reaction, what do we call the substances you start with and the substances you end with?
Reactants and products.