What is a form of matter that has characteristic chemical properties? Hint S
Substance
The process by which the information on mRNA is converted into a specific sequence of amino acids to form a protein?
Translation
In an atom which particles are found in the nucleus?
Protons and neutrons are found in the nucleus
Gasoline does not dissolve in water. Is it polar or nonpolar? Hydrophobic or hydrophilic?
Non polar, which makes is hydrophobic.
Water is polar so it dissolves other polar things. Anything that dissolves in water is hydrophilic and anything that doesn't is hydrophobic.
Identify the carbohydrate
Na2CO3
C2H6O
C8H16O8
C2HO
C8H16O8
Carbohydrates have carbon atoms and twice as many H atoms as O atoms.
When three fatty acids chemically link to a molecule of glycerol, what kind of lipid is formed?
A triglyceride
A molecule has an amino group, and acid group and a side chain. Which group of biological molecules does it belong?
Amino acid.
The smallest stable unit of all physical matter, which is comprised of protons, neutrons and electrons.
Atoms
A sequence of nucleotide bases on DNA that codes for a macromolecule (such as a protein or RNA)?
Gene
In an atom which particles are found outside the nucleus?
Electrons
Wax paper is covered with a nonpolar substance. Compare water's adhesive attraction to wax paper and its adhesive attraction to a paper towel.
Water's adhesive attraction to wax paper is small compared to its adhesive attraction to a paper towel.
Remember, adhesion measures how attracted water is to another substance. If the substance is nonpolar, water won't be attracted to it. However, water is very attracted to a paper towel. Experiment 2.1
When two monosaccharides are bonded together, what two substances are formed?
A disaccharide and water
What kind of reaction is when three fatty acids chemically link to a molecule of glycerol, what kind of lipid is formed?
dehydration reaction
When two molecules like the one described in the previous question react so that there is a bond between the C in the acid group and the N in the amino group, what do we call the molecule that results?
It is called a dipeptide.
A pure substance that is composed of a unique type of atom.
Element
The illustration of a DNA strand which letter indicates a nucleotide base?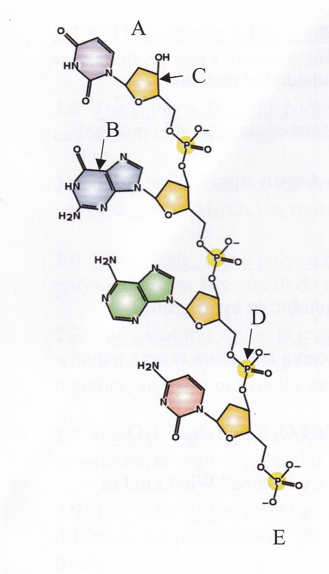
B the nucleotide bases are attached tot he sugars inside the backboone
An atom donates an electron to a different atom. What kind of bond is formed through this process?
Ionic bond. When atoms loose or gain electrons they become charged and are called ions. The opposites charges cause the ions to stay close together.
What is the reaction called when two monosaccharides are bonded?
dehydration reaction
If the fatty acid molecules have double bonds between the carbon atom what kind of fat do they make?
A unsaturated fat.
Like peanut butter it separates.
What do we call it when several such molecules do the same thing?
Several such reactions lead to a polypeptide.
A group of atoms that from a unit with specific chemicals properties. The atoms may be the same or they may be different, depending on the specific chemical involved. Hint M
Molecule
In the illustration of this DNA which letter indicates
is at the 3' end?
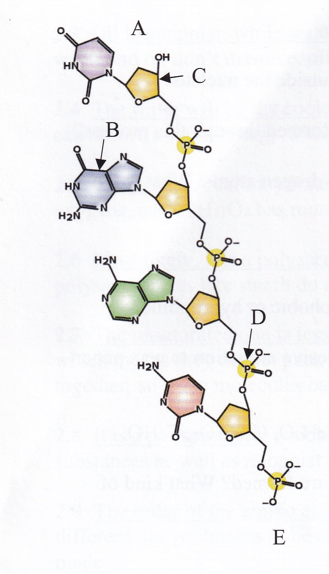
A The 3' end is the one that has a sugar attached to nothing
Natural gas is composed of a carbon atom covalently bonded to four hydrogen atoms. What is the chemical formula?
Covalent = relating to or denoting chemical bonds formed by the sharing of electrons between atom
CH4 In chemical formulas, the subscripts tell you the number of each atom, but there is only one atom, no subscript is used.
Starch is an example of what kind of carbohydrate?
A polysaccharide
Polymer is also correct, but there are many biological polymers. It is best to be specific.
This diagram represents a phospholipid
Is the pink or blue hydrophilic or hydrophobic?

The head (pink) is hydrophilic.
The tails (blue) are hydrophobic.
The in-between close to the head where the tails meet are a transition between the two.

What do we call the bond that links he C and N?
The bond is a peptide bond.
You could say "protein" instead of polypeptide, but there are some polypeptides that are not proteins.
A substance that contains two more more atoms from different types of elements held together by chemical bonds. Hint C
Compound
In the illustration of this DNA which letter indicates
is at the 5' end?

E The 5' end has a phosphate attached to nothing.
Compare hydrogen bonds to covalent bonds.
Covalent bonds are stronger than hydrogen bonds. Also, covalent bonds exist within a single molecule, while hydrogen bods exist between two separate molecules.
What is the primary structure of a protein?
It is the order in which the protein's amino acids link together.
Having a tendency to attract water?
Hydrophilic
In the illustration of this DNA which letter indicates
a sugar?

C The pentagram represents the sugar deoxyribose.
Two proteins are both composed of seven different amino acids. Furthermore the number of each of those amino acids is the same in both proteins. Are the proteins the same? Why or Why not?
Not necessarily the same. Even if they have all the same amino acids, those amino acids must be linked together in exactly the same order if they are the same proteins. In other words, they must have the same primary structure to be the same protein.
Having a tendency to repel water?
Hydrophobic
In the illustration of this DNA which letter indicates
a phosphate?

D The group that is made of P's and O's is the phosphate group.
The pH of the solution in your stomach, called gastric juice is 1. Is gastric juice and acid, base or neither?
Acid
A property in which the molecules of a substance are attracted to its own molecules. Hint C
Cohesive
What kinds of bonds hold DNA's double helix together?
Hydrogen bonds hold the double helix together.
The pH of water is 7. Is water an acid, base or neither.
neither
A property in which molecules of a substance are attracted to other similar substances? Hint A
Adhesive
One strand of DNA has the following sequence of nucleotide bases
C-G-T-A-A-G
What sequence is on the strand on the opposite side of the double helix?
C-G-T-A-A-G
G-C-A-T-T-C
G connect same shape C
A's connect to T AT AT's like Starwars.
The Ph of the ocean water is 8. Is it acid, base or neither?
Base or Basic.
pH scales says 7 is neutral
above 7 basic
below 7 acid
A molecule composed of carbon, hydrogen and oxygen in which there are twice as many hydrogen atoms as oxygen atoms?
Carbohydrate
One strand of DNA has the following sequence of nucleotide bases
C-G-T-A-A-G
If the sequence above were transcribed, what would be the sequence on the mRNA
C-G-T-A-A-G
G-C-A-U-U C
G connect to C's (Same shape)
U replaces the T in RNA AU AU not star wars.
An enzyme is a specific type of what kind of molecule?
A specific type of protein.
A hydrophobic macromolecule that readily dissolves in nonpolar environments?
Lipid
One strand of DNA has the following sequence of nucleotide bases
C-G-T-A-A-G
How many amino acids would that strand of mRNA be translated into?
It would be translated into two amino acids.
Remember, it takes three nucleotide bases to code for an amino acid. Since there are six in the sequence, it can only code two.
What is an enzyme's function?
It function is to enable chemical reactions to occur at a speed that permits life.
A complex polypeptide that play a role in the chemistry of an organism?
Protein
Polypeptide: a linear organic polymer consisting of a large number of amino-acid residues bonded together in a chain, forming part of (or the whole of) a protein molecule.
Nucleotide bases in RNA are attaching to a strand of DNA. IS this par of transcription or translation?
Transcription: involves building an mRNA based on the DNA sequence. This requires the nucleotide bases in RNA to attach to the bases on DNA.
The molecules involved in the making of proteins and the passage of traits from one generation to the next?
Nucleic acids
An mRNA molecule is at a ribosome.
Is this part of transcription or translation?
ribosome: a minute particle consisting of RNA and associated proteins found in large numbers in the cytoplasm of living cells. They bind messenger RNA and transfer RNA to synthesize polypeptides and proteins.
The process by which DNA is copied to produce two duplicate double helixes?
Replication
A sample of sugar is all right handed. Was it produced b a living organism or by processes that were not guided by a living organism?
From a living organism: if a sample has all right- or left-handed molecules, it was made by a living organism.
The process by which DNA is used to produce messenger RNA?
Transcription
What four steps must be explained by those who want to believe life arose through chemical evolution. Have any of them been explained?
It must explain:
1. The appearance of the correct organic macromolecules.
2. The polymerization of these macromolecules
3. How protocells (pre-curses to cell) develop.
4. The development of the first cell.
So far, no chemical evolution mechanism can explain any of these steps