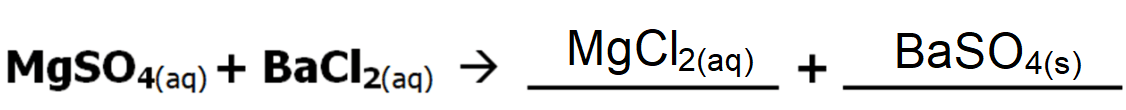Name the 3 particles that make up and atom
Protons
Neutrons
Electrons
How many valence electrons does Silicon (atomic #14) have?
4
Would Fluorine (#9) rather give or take electrons to have a full outer shell?
take electrons
In ionic compounds, atoms are transferred. In covalent compounds, atoms are ________.
Why is it difficult to predict the properties of Boron (#5)?
It is a metalloid (properties of both metals and nonmetals)
What are the charges and mass of each particle that makes up an atom?
Protons: (+) 1 amu
Neutrons: (0) 1 amu
Electrons: (-) 0 amu
List two properties that most all metals on the periodic table share.
Malleable
Shiny
Reactive
Conductive
What charge would a Magnesium Ion have?
2+
What sort of elements on the periodic table form covalent bonds?
nonmetals
Is this compound Ionic or Covalent: CuCl2
Ionic (metal and nonmetal)
In a neutral atom, what element has 8 electrons?
Oxygen
How many energy levels the atom has
Draw the lewis dot structure for the ionic compound: Sodium Chloride
(hint: use brackets and charges)
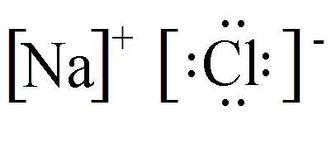
How many electrons are shared in a Bromine (Br2) (#35) molecule?
2 electrons are shared
On the periodic table the mass number is not a whole number. How can that be the case?
It is the average of all the masses of that particular element/atom.
Atoms vary in the amount of neutrons they have, so when you find the average, it won't be a whole number.
What is the "job" of the electrons?
They allow atoms to combine with other atoms.
What do all Nobel Gases have in common?
Fuller outer energy levels (nonreactive)
How many ions of Potassium (#19) will there be in an ionic bond between Potassium and Nitrogen (#7)?

Draw the lewis dot structure of a covalent compound that is composed of Oxygen & Flourine
(Hint: "venn diagram"or "bar diagram")
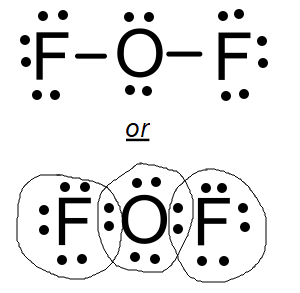
2 part question
The following is an example of a double displacement precipitate reaction. What will the products be?
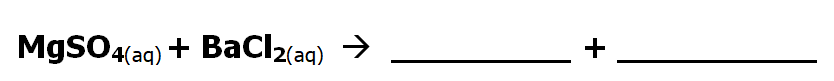
Hint: "switch partners" & check charges
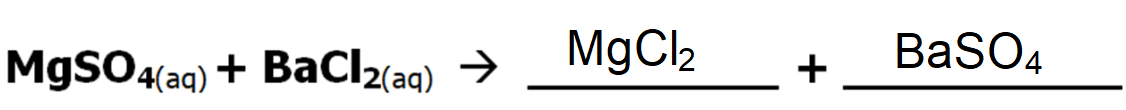
How many neutrons are in isotope Calcium-45?
5 neutrons
Why is calcium (#20) more reactive than Magnesium (#12)?
Valence electrons are further away from the nucleus.
What holds the atoms in an ionic bond together?
Their opposite charges (like a magnet)
What is the name of this covalent compound: H2S
hydrogen sulfide
hydrogen monosulfide
dihydrogen monosulfide
dihydrogen sulfide
dihydrogen monosulfide
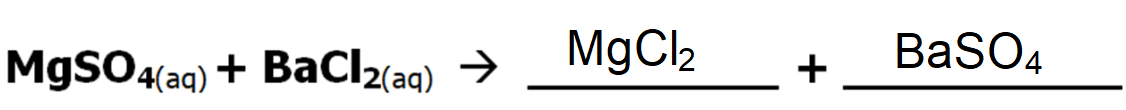
What product will form a precipitate? Or will any precipitate form?