It needs to be kept constant in Boyle's Law
What is Temperature
Driving through a desert on a summer day can see the tires of your car get shredded.
What is due to expanding volume.
One mole of all gasses occupy this volume at STP
What is 22.4 L
Three factors that affect gas pressure.
What are the temperature, the volume and the number of particles.
Ideal gases
What are do not exist.
What is Boyles' Law
The phenomenon that the density of air in a hot air balloon decreases causing it to float up.
What is due to Charle's Law.
Temperature and pressure are directly proportional under this condition.
What is at constant volume.
The relationship between the number of molecules and the volume of a gas: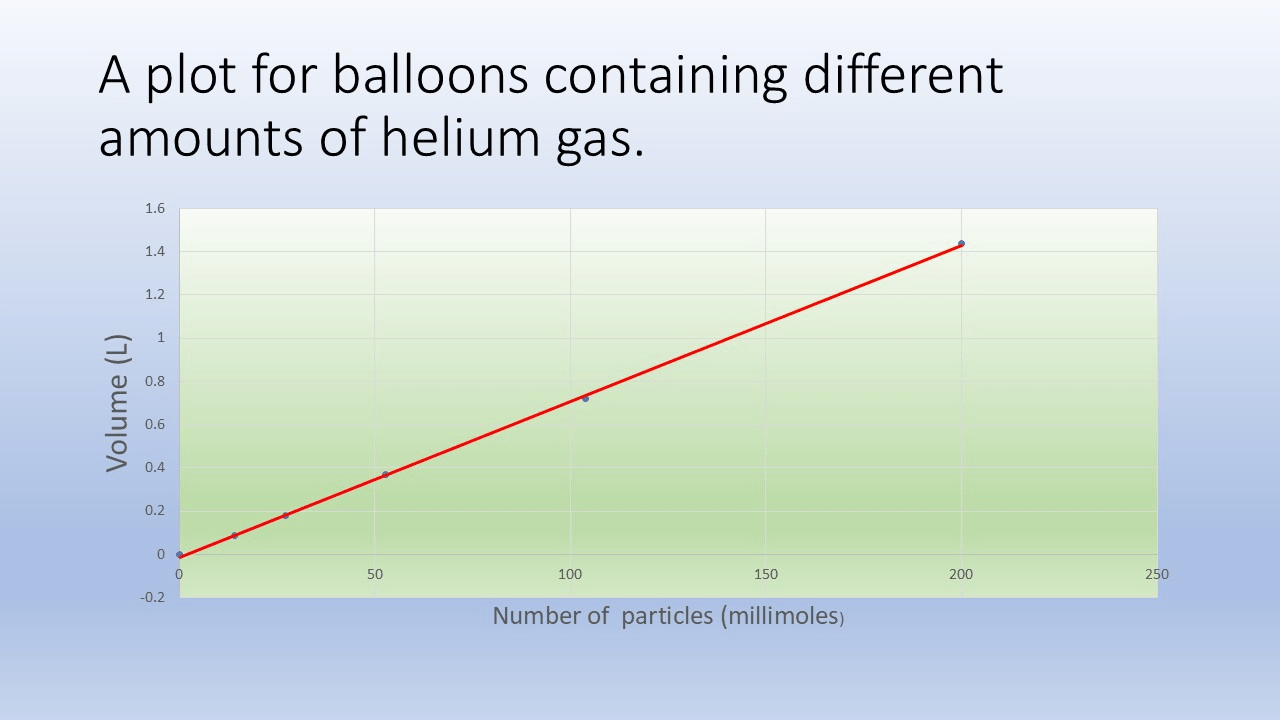
What is Avogadro's principle.
The combined gas law describes this.
What is the relationship between temperature, pressure and volume of a gas.
A helium balloon occupies 2.5 L volume at 1 atm. What will be its volume when the same balloon is taken down to a depth of 10.5 meters under the sea where the pressure is 2 atm?
What is 1.25 L.
The can of soda pop explodes when it is frozen.
What is due to an increase in the volume of frozen water the pressure increases.
The number of particles in gases at standard temperature and pressure are proportional to this gas quantity.
What is the volume.
The mathematical expression when we include the number of particles in relating the pressure, volume and temperature of a gas.
What is the combined gas law.
The standard pressure at the beach.
What is 1 atm.
Twice as many oxygen and nitrogen molecules are inhaled during this activity.
What is a SCUBA dive.
At constant pressure the volume occupied by a fixed amount of gas is directly proportional to its absolute temperature.
What is Charles' law.
When temperature is absolute zero, the volume of a gas becomes zero to maintain the relationship: PV = k!
What is false.
The number of moles constituted by 1.50x1024 particles.
What is 2.5 moles.
The mass of 22.4 L of Hydrogen gas at standard temperature and pressure.
What is 2.016 g.
Your clothes feel tight because the contents of your stomach expand during this.
What is during a flight when the airplane reaches cruising altitudes of 10,000 m.
A gas balloon has a volume of 5 L, at 25 0C, The volume of this balloon changes to this when the temperature is raised to 100 0C, at constant temperature.
What is 20L.
A cylinder with a moving piston with air at a pressure of 100 kPa registers a volume of 0.75 L. When the pressure is increased to 300 kPa, the new volume will be-------------, if the temperature does not change.
What is 0.25 L.
A cylinder contains 2 moles of nitrogen, and 2 moles of oxygen. When you count the total number of particles you find that your cylinder has -------------- particles.
What is 2.408 x 1024 particles or 4 mols.
The pressure of an ideal gas doubles when the temperature is changed.
What is two times the initial temperature in Kelvins.