This is the simplest form of matter, made of only one kind of atom.
What is an element
This type of substance is made out of components that are uniformly spread out?
Homogenous mixture
This allows water molecules to stick to each other
what is cohesion
How can the periodic table you identify elements
The periodic table helps you identify elements by showing their atomic number (protons), symbol, and grouping them by similar properties.
This is value that determines if a substance is acidic or basic
what is pH
How do we know if that conservation of mass holds true for a reaction?
If the number of atoms and the mass is conserved from the reactants to products
Oxygen (O₂), gold (Au), and iron (Fe) are all examples of this.
What are elements
You can see the layers of components in this type of mixture
What is heterogenous mixture
This allows water to stick to other surfaces
what is adhesion
Identify the elements in the following:
4Fe + 3O2 → 2Fe2O3
Fe = iron
O = oxygen
What makes a substance an acid? How do we know if it is a strong acid or not?
an acid is any substance with a pH value below 7. The lower that pH value, the stronger the acid.
How many oxygens are in the reactants of the following equation?
12H₂S + 6CO₂ → C₆H₁₂O₆ + 6H₂O + 12S
12 Oxygens
Water (H₂O) and carbon dioxide (CO₂) are examples of this type of pure substance.
What are compounds
Which represents a homogenous mixture and heterogenous mixture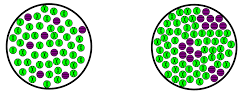
Left = homogenous
Right = heterogenous
what is Capillary Action
Matt mixes baking soda(NaHCO₃) and vinegar (CH₃COOH) producing a white/cloudy precipitate of sodium acetate (CH₃COONa). What elements are in the precipitate?
CH₃COOH + NaHCO₃ → CH₃COONa + H₂O + CO₂
C = carbon
H = hydrogen
O = oxygen
Na = sodium
What makes a substance a base? How do we know if its a strong or weak base?
A base is a substance that has a pH above 7. The higher the pH value the stronger the base
What is the total amount of irons before and after the reaction in the following equation?
4Fe + 3O2 → 2Fe2O3
4 irons
Describe the difference between an element and compounds
What type of mixture is this example?
What is heterogenous
Water climbing up a stem of a tree is the result of what water properties
cohesion and adhesion
K = potassium
N = Nitrogen
O = Oxygen
P = Prosperous
Ni = Nickle
Cl = Chlorine
It is a strong acid and strong electrolytes
A student combines methane with oxygen to create a combustion reaction. The student produces 68 grams of carbon dioxide and 2 grams of water. The equation is as followed:
CH₄+ 2O₂ → CO₂+ 2H₂O
If the student used 27 grams of methane, how much oxygen is needed for the reaction?
43 grams
Which represents an element and which are compounds?
X = elements
Y = compounds
Which of the following are heterogenous mixtures?
1. Sand and water
2. Sugar and water
3. Oil and vinegar
4. Iron fillings and sulfur powder
1, 3, 4
John is observing water droplets on the surface of a leaf after a rainstorm. She notices that the droplets are rounded and do not spread out.
What property of water is responsible for this behavior?
Surface Tension
What elements are present in the following equation?
SnO2 + 2H2 → Sn + 2H2O
Sn = Tin
O = Oxygen
H = Hydrogen
what are characteristics of bases
can taste bitter, feel slippery and turns indicators blue
6CO₂ + 6H₂O + Light energy → C₆H₁₂O₆ + 6O₂
If a plant uses 21 grams of carbon dioxide and 79 grams of water, and produces 64 grams of oxygen, how much glucose is also produced
36 grams
A compound
This is the main difference between a compound and a mixture.
compounds are chemically combined, mixtures are physically combined?
Describe the phenomena in this picture 
Adhesion pulls the water molecules upward through the spaces between the paper towel fibers while cohesion keeps them together.
Identify the elements in the following equation
12H₂S + 6CO₂ → C₆H₁₂O₆ + 6H₂O + 12S
H = Hydrogen
S = Sulfur
C = Carbon
O = Oxygen
A student puts vinegar on a litmus paper and it turns red. Is the vinegar an acid or base?
an acid
What can you say about the number of carbon atoms from the beginning to the end of this reaction?
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O + ATP