Write the dissociation equation for KOH.
KOH --> K+1 + OH-1
Write the balanced chemical equation for ...
HCl + NaOH -->
HCl + NaOH --> H2O + NaCl
Litmus paper is blue and phenolphthalein is pink. Is the solution acidic, basic, or neutral?
Basic
HNO3
Is it a strong acid, weak acid, strong base, weak base, or neutral?
strong acid
What is the pH of a 0.001 M solution of HCl?
3
Write the dissociation equation for HBr.
HBr + H2O --> H3O+1 + Br-1
Write the balanced chemical equation for ...
H2SO4 + 2 KOH -->
H2SO4 + 2 KOH --> 2 H2O + K2SO4
A basic solution contained phenolphthalein when acid was added to it. During this titration, the solution's color changed from ________ to ________.
pink, clear
NaOH
Is it a strong acid, weak acid, strong base, weak base, or neutral?
strong base
What is the concentration of an NaOH solution with a pH of 13?
0.1 M
Write the dissociation equation for H3PO4.
H3PO4 + H2O <-> H3O+1 + H2PO4-1
Write the balanced chemical equation for ...
HBr + Fe(OH)3 -->
3 HBr + Fe(OH)3 --> 3 H2O + FeBr3
What do we call this equipment and what is its purpose during a titration lab?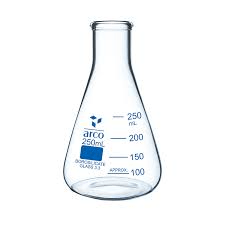
Flask
Container for mixing acid and base
HCN
Is it a strong acid, weak acid, strong base, weak base, or neutral?
weak acid
It takes 30 mL of 0.1 M NaOH to completely neutralize 120 mL of HCl. What was the Molarity of the HCl?
0.025 M
Write the dissociation equation for CH3CH2COOH.
CH3CH2COOH + H2O <-> H3O+1 + CH3CH2COO-1
Write the balanced chemical equation for ...
H2SO4 + Sr(OH)2 -->
H2SO4 + Sr(OH)2 --> 2 H2O +SrSO4
What do we call this equipment and what is its purpose during a titration lab?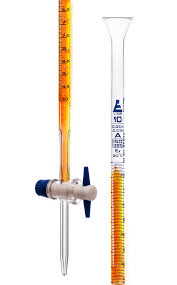
Buret
Measuring volume of solution added
KF Is it a strong acid, weak acid, strong base, weak base, or neutral?
weak base
It takes 8 mL of 0.03 M HCl to completely neutralize 250 mL of NaOH. What was the initial pH of the NaOH solution?
approximately 3
Write the dissociation equation for CH3CH2NH2
CH3CH2NH2 + H2O <-> CH3CH2NH3+1 + OH-1
Write the balanced chemical equation for ...
H3PO4 + Cu(OH)2 -->
2 H3PO4 + 3 Cu(OH)2 --> 6 H2O + Cu3(PO4)2
volume
neutralize another solution; or
make moles acid = moles base
FeCl3
Is it a strong acid, weak acid, strong base, weak base, or neutral?
weak acid
You spilled 20 mL of 0.008 M HNO3. How much 0.001 M KOH must be added to this spill in order to change the pH to 7?
160 mL