How many valence electrons does oxygen have?
6
How many total electrons does sodium (Na) have?
11
Write the electron configuration of carbon.
Carbon: 1s² 2s² 2p².
Which rule states that no two electrons in the same orbital can have the same spin?
Answer: Pauli Exclusion Principle
What causes the colors seen in fireworks?
Firework colors come from electrons moving between energy levels and releasing photons.
DOUBLE: Draw the Lewis dot diagram for chlorine.

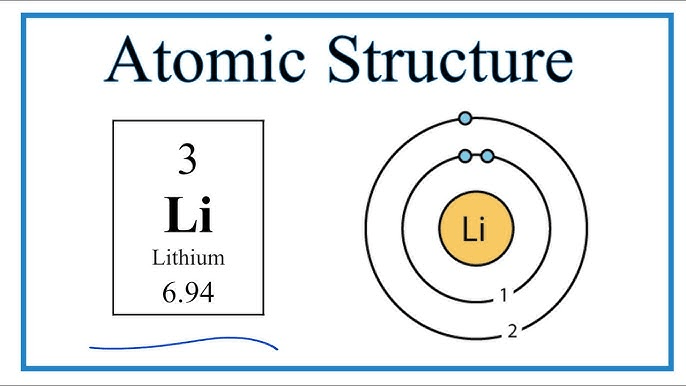
Draw the Bohr model for lithium.
What is the noble gas shorthand for sulfur?
Sulfur shorthand: [Ne] 3s² 3p⁴.
QUADRUPLE: What is the shape of a p orbital ?
Dumbbell-shaped / two lobes
DOUBLE: Which has the higher energy: red light or blue light?
Blue
DOUBLE: Which group on the periodic table has 2 valence electrons?
Group 2
TRIPLE: Which energy level is being filled in period 3 (Row 3) of the periodic table?
Energy Level 3
Write the e-config for nitrogen.
1s² 2s² 2p³
Name this orbital shape.
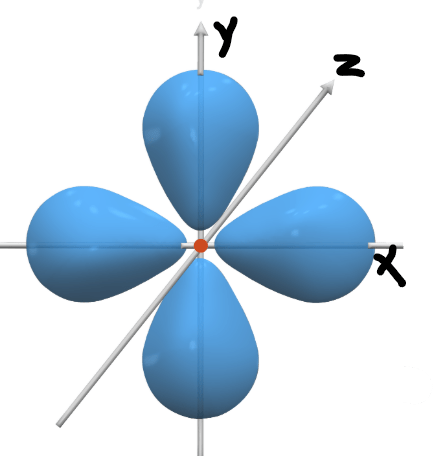
dX2y2
TRIPLE: Spit the names of 10 elements that rhyme!
Answers vary.
Which group on the periodic table includes elements with a full outer valence shell?
Group 8 - Noble Gases (last column to the right)
Compare the Bohr models of nitrogen and fluorine — what’s different about their outer shells?
Nitrogen has 5 valence electrons, fluorine has 7 → difference in outer shell electron count.
QUADRUPLE: Write the e-config for Francium.
1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁶6s²4f¹⁴5d¹⁰6p⁶7s¹.
Which rule explains why electrons occupy separate orbitals of the same energy before pairing up?
Answer: Hund’s Rule
Why are GAMMA rays so dangerous?
Gamma rays are higher frequency, shorter wavelength, and higher energy, so they penetrate tissue and damage cells more than microwaves.
DOUBLE: Name two elements that would have the same number of dots in their Lewis Dot Diagram.
Answers vary.
TRIPLE: Explain why the Bohr model breaks down for larger atoms.
The Bohr model fails for large atoms because electrons don’t orbit in fixed paths — quantum mechanics describes orbitals as regions of probability.
Also, more than 8 electrons occupy levels beyond energy level 2/3 for d and f sub levels.
Write the electron configuration for bromine using the noble gas shortcut.
[Ar] 4s² 3d¹⁰ 4p⁵.
Draw the orbital diagram for oxygen (O, atomic #8).
(Answer: 1s ↑↓, 2s ↑↓, 2p ↑↓ ↑ ↑)
TRIPLE: Why does the 4s orbital fill before the 3d orbital in electron configurations?
Answer: Because 4s is slightly lower in energy than 3d, according to the Aufbau principle