What is the general formula for alkanes?
CnH2n+2
1) What is a formal charge? 2) How do you calculate formal charge?
1) It's a theoretical charge on atoms in a molecule.
2) Formal charge = valence electrons – (number of lone-pair electrons + ½ number of bonded electrons) or
FC = VE - (no. of lone pairs + number of bonds)
CH3F has a dipole moment, explain how a dipole moment occurs.
This occurs when atoms in a molecule have different electronegativities. This causes the atoms to surround one atom more giving it a slight negative charge and the other a slight positive charge.
What is the difference between a molecular formula and a structural formula?
Molecular formula gives the number of atoms present in a compound where as a structural formula shows how the atoms are bonded together with their lone pairs.
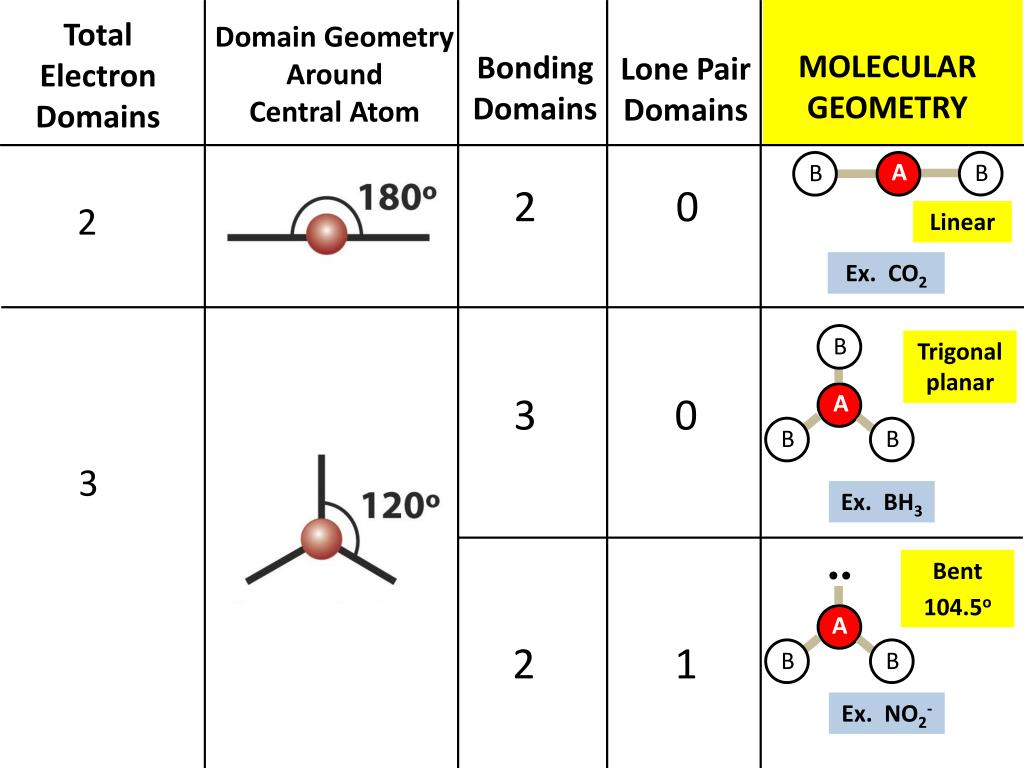
What is the molecular geometry of a compound?
the arrangement of atoms in a molecule.
Which intermolecular force is primarily responsible for the interactions among alkane molecules?
Van der whaals forces
Find the formal charge for each atom in the compound below

H - +1
C- 0
N - 0
Draw the molecular geometry of BF3 and state whether it has a dipole moment.
no dipole moment
Draw the structural formula for CH3CHO.
What molecular geometry does BH3 have? State whether it is polar or non- polar.
trigonal planar and non- polar
When one compares the densities of hexane and water, one finds:
A) that hexane is less dense than water.
B) that hexane is more dense than water.
C) that these two compounds have the same density.
D) that the relative densities of two immiscible compounds cannot be measured.
A
What is the formal charge for carbon and nitrogen in the compound below: (left to right)

C - 0
N- +1
N : -1
What is the formula for dipole moment?
(μ) = e x d
Write the condensed formula for the following molecule:

CH3(CH2)4CH3
The H2S molecule is _________ and ________.
bent and polar
The following molecule contains how many 1°, 2°, and 3° hydrogens?

1o - 9
2o - 2
3o - 1
Give the formal charges on all atoms in the compound below: (left to right)
O: 0
S: +1
O: -1
Which of the following covalent bonds has the largest dipole moment?
A) C-C B) C-O C) H-N D) H-F
D
Write the condense formula for the compound below:

CH3COOCH2CH3
Work out the molecular geometry of C2H6 and state whether it is polar or non-polar.
tetrahedral and non-polar
Name the two major steps in the refining of crude oil into usable hydrocarbon products.
1) Fractional distillation separates mixtures based on boiling point.
2) Catalytic/ Hydro cracking - a catalyst is added together with heat and hydrogen (in case of hydro cracking) to break up long chain alkanes into short chains.
Give the formal charge of nitrogen:

N - +1
Which of the following molecules does not exhibit a net dipole moment of zero?
A) CO2 B) CH4 C) CCl4 D) H2O
D
Draw the lewis structure for CH3CN

How to determine the molecular shape of a compound?
1) Draw molecule's lewis structure.
2) Count the number of bonds to atoms and lone pairs.