K on the periodic table of elements represents the element -
A. Calcium
B. Nitrogen
C. Potassium
D. Carbon
C. Potassium
Which of these is a solution
A. Salt
B. Water
C. Lemonade
D. Sugar
C. Lemonade
An electron has a _______________ charge
A. Positive
B. Neutral
C. Negative
D. Cloud
B. Negative
Which of these will change solid iron to a liquid?
A. raising the air pressure
B. increasing its temperature
C. crushing the solid iron
D. adding water to the iron
B. increasing its temperature
Which of the diagrams best shows the arrangement of molecules in a solid? 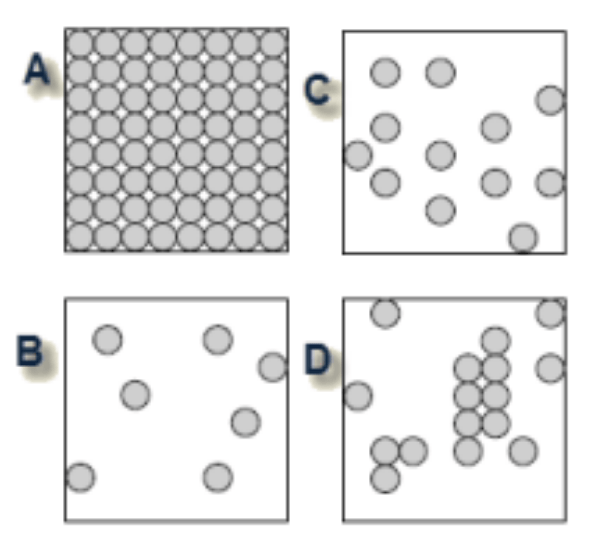
A
Identify the compound:
Water
Helium
Potassium
Oxygen
Water
Which of these is a mixture
A. Salt
B. Helium
C. Chocolate Milk
D. Chex-mix
D. Chex-mix
An proton has a _______________ charge
A. Positive
B. Neutral
C. Negative
D. Cloud
A. Positive
Which of these will happen if the temperature of a metal pan is increased?
A. the pan will begin to lose heat
B. the molecules of the pan will move faster
C. the metal will change into another metal
D. the pan will contract
B. the molecules of the pan will move faster
What will happen if the lid is removed from a container that holds helium gas?
A. The gas will expand and escape from the container
B. The gas will slowly change back into a liquid
C. When light hits the gas, it will change colors
D. Gravity will keep the gas in the container
A. The gas will expand and escape from the container
Identify the elements:
Water
Helium
Potassium
Salt
A student makes a fruit drink by stirring a powdered mix into cold water. Why is the fruit drink a solution?
A. The powder dissolves in the water
B. The water changes color
C. The student stirs the water
D. The water in the proper temperature
A. The powder dissolves in the water
An Neutron has a _______________ charge
A. Positive
B. Neutral
C. Negative
D. Cloud
B. Neutral
When two or more elements combine to form a new substance
A. Compound
B. Molecule
C. Atom
D. Element
A. Compound
When ice cream is left out of a freezer, the ice cream changes from a -
A. Solid to a gas
B. Gas to a liquid
C. Solid to a liquid
D. Liquid to a gas
C. Solid to a liquid
Substance that cannot be broken down into simpler substance
A. Compound
B. Molecule
C. Atom
D. Element
D. Element
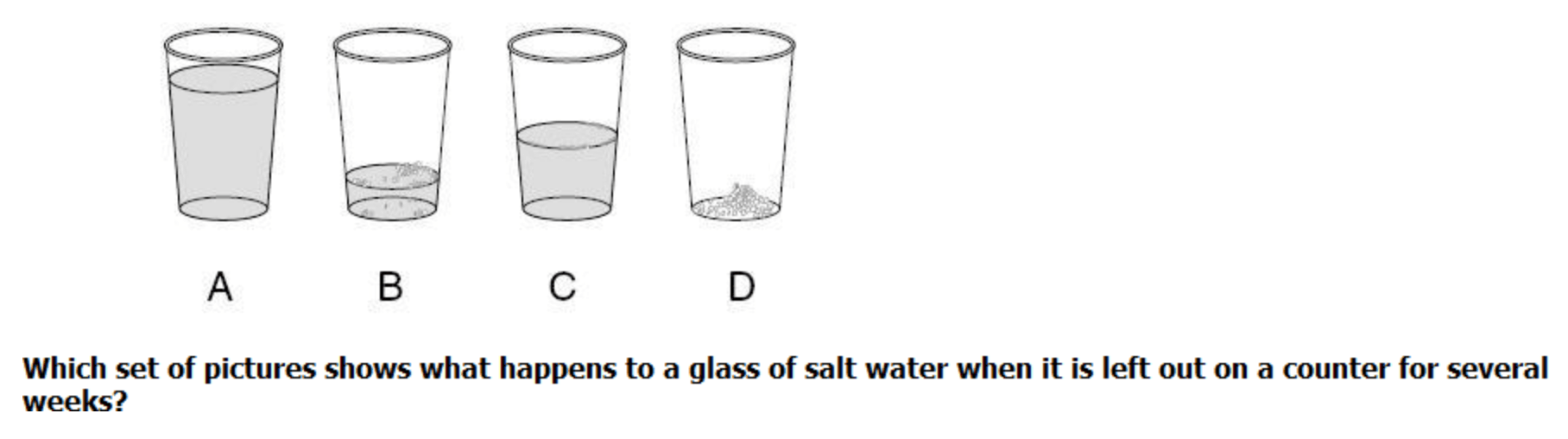
1. B - A - C - D
2. A - C - B - D
3. C - D - A - B
4. D - B - A - C
2. A - C - B - D
Smallest part of a compound
A. Compound
B. Molecule
C. Atom
D. Element
B. Molecule
Which of the diagrams best shows the arrangement of molecules in a liquid?
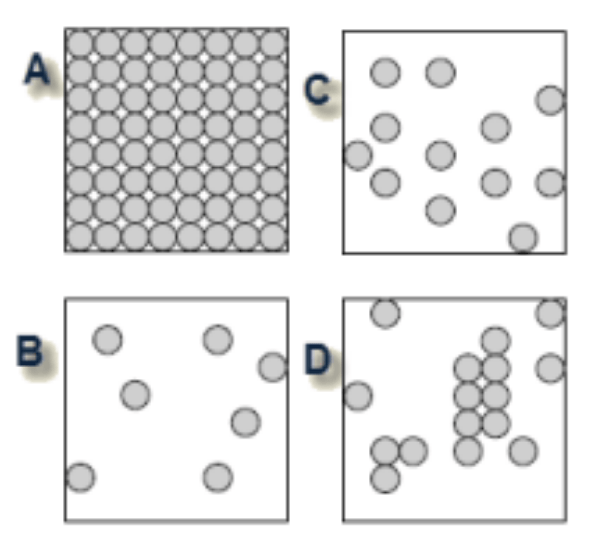
C
Oxygen, nitrogen, and carbon dioxide may be grouped together because at room temperature they are all a —
A. Solid
B. Liquid
C. Gas
D. Colloid
Gas
Water, ice, and steam are alike because they -
A. are the same compound
B. have the same shape
C. look the same
D. feel the same
A. are the same compound
All __________ are mixtures, but not all mixtures are solutions.
A. compounds
B. solutions
C. elements
D. mixtures
B. solutions
Smallest part of an element that skills had the physical properties of the element
A. Compound
B. Molecule
C. Atom
D. Element
C. Atom
Which of the following changes is possible with the addition of heat?
A. liquid water changes to ice
B. water vapor changes to ice
C. water vapor changes to liquid water
D. ice changes to liquid water
D. ice changes to liquid water
Which of the diagrams best shows the arrangement of molecules in a gas?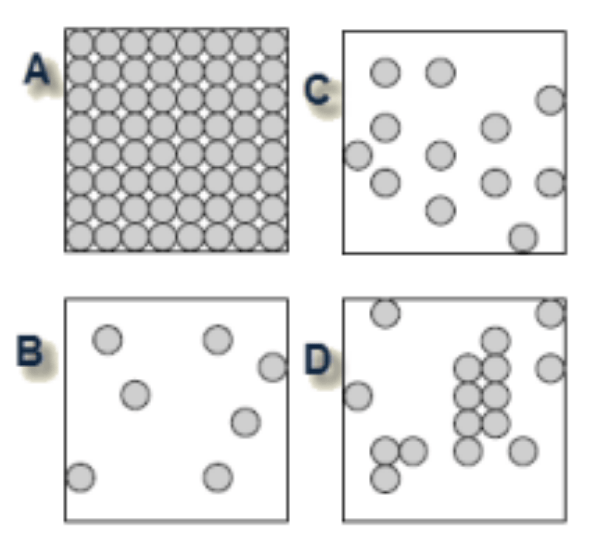
B