The tiny pieces that all matter is made of.
Can rust be created without fertilizer?
No
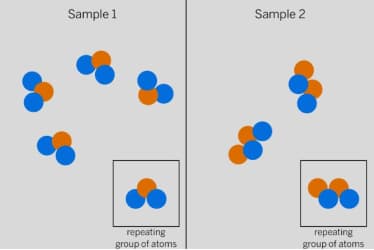
These substances will have __________ properties.
different
The temperature at which a substance changes from the solid phase to the liquid phase is
Melting point
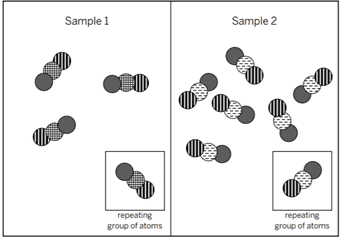
The diagram shows the repeating groups of atoms that make up two samples. The properties will be ________. I know this because ________.
different; the molecules are made of different types of atoms
A group of atoms joined together in a particular way
What is a molecule?
What two substances created rust?
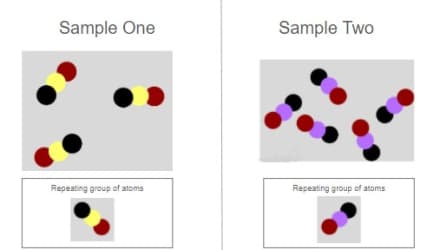
These substances have the exact same properties. True or False?
False
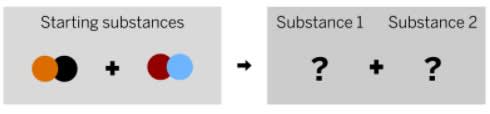
What is a possible product these reactants can form?
red, orange
blue, black
red, black
blue, orange
During a chemical reaction, atoms cannot be ______ or ______.
Created or destroyed
A process in which atoms rearrange to form new substances
Chemical Reaction
What was the reddish-brown substance in the water in Westfield?
Rust
Evidence(s) that a chemical reaction occurred is(are)
The properties change (color, smell, boiling point, etc.)
During a chemical reaction, ______ of the atoms that make up the reactants rearrange to form the products.
All

 devorah is a food chemist, and she is developing a chemical to help foods stay fresh longer. She mixed a red liquid substance and a colorless solid substance together in a sealed container. The diagram above shows the repeating groups of atoms that make up the two starting substances.
devorah is a food chemist, and she is developing a chemical to help foods stay fresh longer. She mixed a red liquid substance and a colorless solid substance together in a sealed container. The diagram above shows the repeating groups of atoms that make up the two starting substances.
After mixing, Devorah found two white substances in the sealed container. (Nothing had escaped.) Which of the diagrams to the left shows the repeating groups of atoms that make up the ending substances?
C
Something that can be observed about a substance, such as color, smell or boiling point.
Property
How did the tokens represent a chemical reaction?
By organizing them by color for each type of atom and showing how they rearranged during the chemical reaction
What can cause an chemical reaction?
Add liquid, heat, cold, or mix
Can substances change into different substances?
yes, because atoms can be rearranged to form different substances
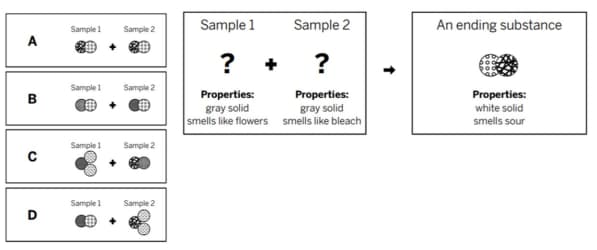
Mr. Cosgrove mixed two samples together: a gray solid that smells like flowers and a gray solid that smells like bleach. He analyzed the results and found two ending substances. One of the ending substances (shown in diagram) was a white solid, made up of the repeating group of atoms shown above. Which of the diagrams shows the repeating groups of atoms that make up the reactants?
D
An ending substance that is made during a chemical reaction.
Product
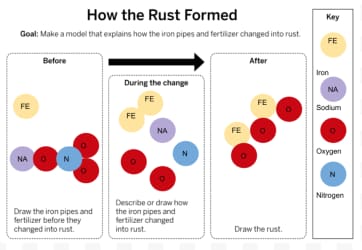
How was rust created? Which atoms were involved?
Hint: Which atoms were used to make up the rust? Where did those atoms come from?
A chemical reaction caused the atoms from the iron pipes and fertilizer to rearrange. Iron and oxygen combined to form the rust.
In this chemical equation, which is one of the products? 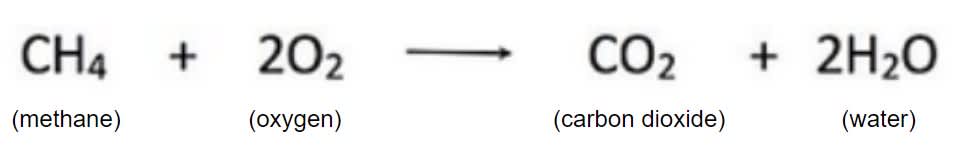
Water
Carbon Dioxide
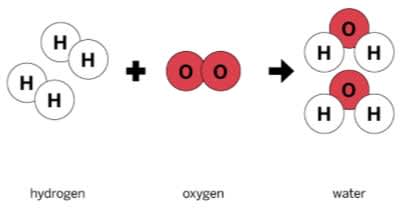
In the reaction shown, hydrogen and oxygen are the _______.
Reactants
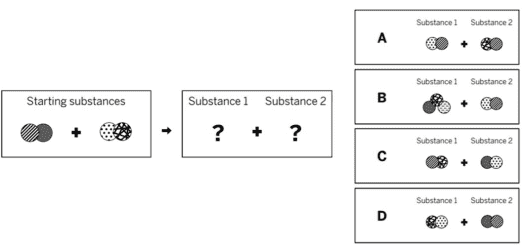
Ms. Schoolcraft is a chemist who is making a chemical to add to swimming pools in order to make the water safer. She mixed two solid substances together in a sealed container. The diagram above shows the repeating groups of atoms that make up the two starting substances. After mixing, Ms. Schoolcraft found two liquid substances in the sealed container. (Nothing had escaped.) Which of the diagrams to the right shows the repeating groups of atoms that make up the products?
c
