These are tiny pieces that all matter is made of
What are Atoms?
What was the reddish-brownish substance in the water in Westfield?
Rust
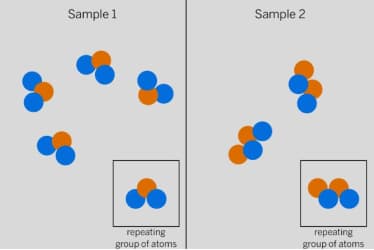
Do the repeating groups of atoms in Samples 1 and 2 have the same properties? Explain!
No, they have different properties because they have a different combination of atoms.
The temperature at which a substance changes from the solid phase to the liquid phase is this property.
What is Melting point?
An object, diagram, or computer program that helps us understand something by making it simpler or easier to see.
What is a Model?
Which Claim is correct?
Claim 1: The iron pipes changed into rust.
Claim 2: The fertilizer changed into rust.
Claim 3: The iron pipes and the fertilizer changed into rust.
What is Claim 3?
What is some evidence that a chemical reaction occurred?
Hint: What are the different properties a substance can have?
Color, Smell, Boiling or Melting Point
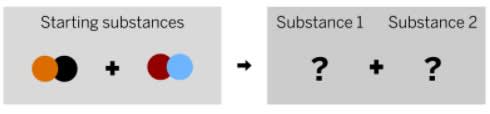
What is a possible product these reactants can form?
red/orange + blue/black
or
red/black + blue/orange
This is the process in which atoms rearrange to form new substances
What is a Chemical Reaction?
What two substances created rust?
What is an example of a Lab Condition that molecules must have to have a chemical reaction?
In water, room temperature, electricity
During a chemical reaction, ______ of the atoms that make up the reactants rearrange to form the products.
a. Some
b. All
C. None
All
the beginning and ending substances that are made during a chemical reaction.
Reactants and Products
How did the tokens represent a chemical reaction?
By organizing them by color for each type of atom and showing how they rearranged during the chemical reaction
What can cause an chemical reaction?
Add liquid, heat, cold, or a combination
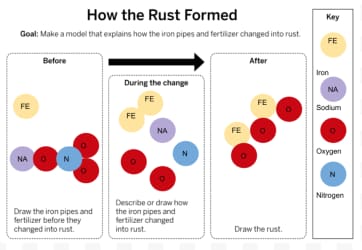
Can substances change into new, different substances? Explain!
Yes, because atoms can be rearranged to form different substances.
Something that can be observed about a substance, such as color, smell or boiling point.
What is a Property?
How was rust created? Which atoms were involved?
Hint: Think of the tokens
A chemical reaction caused iron and oxygen to combine from the fertilizer running through the pipes
Explain what occurred in this chemical reaction 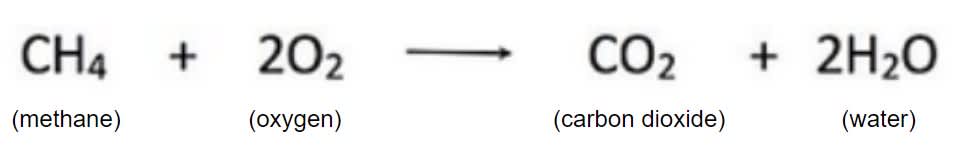
The atoms in the methane (1 carbon, 4 hydrogen) reacted to the atoms in oxygen (2 atomic pairs of 2 oxygen). After the chemical reaction, the carbon atoms combined with one of the oxygen molecules to create carbon dioxide (CO2), while the hydrogen atoms combined with the other oxygen molecule to create 2 water molecules (H2O)
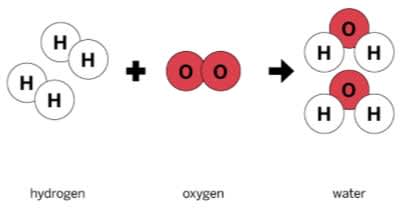
In the reaction shown, hydrogen and oxygen are the _______, while the two H2O are the ____________.
Reactants, Products
