What is an atom?
Atoms are the smallest unit of matter.
What are the 3 building blocks of an atom?
Protons, neutrons, and electrons
Elements are listed in the periodic table by this type of number.
What is atomic number?
What is the difference between a cation and an anion?
Cation - Lose Electrons
Anion - Gaining Electrons
How many groups of elements are there?
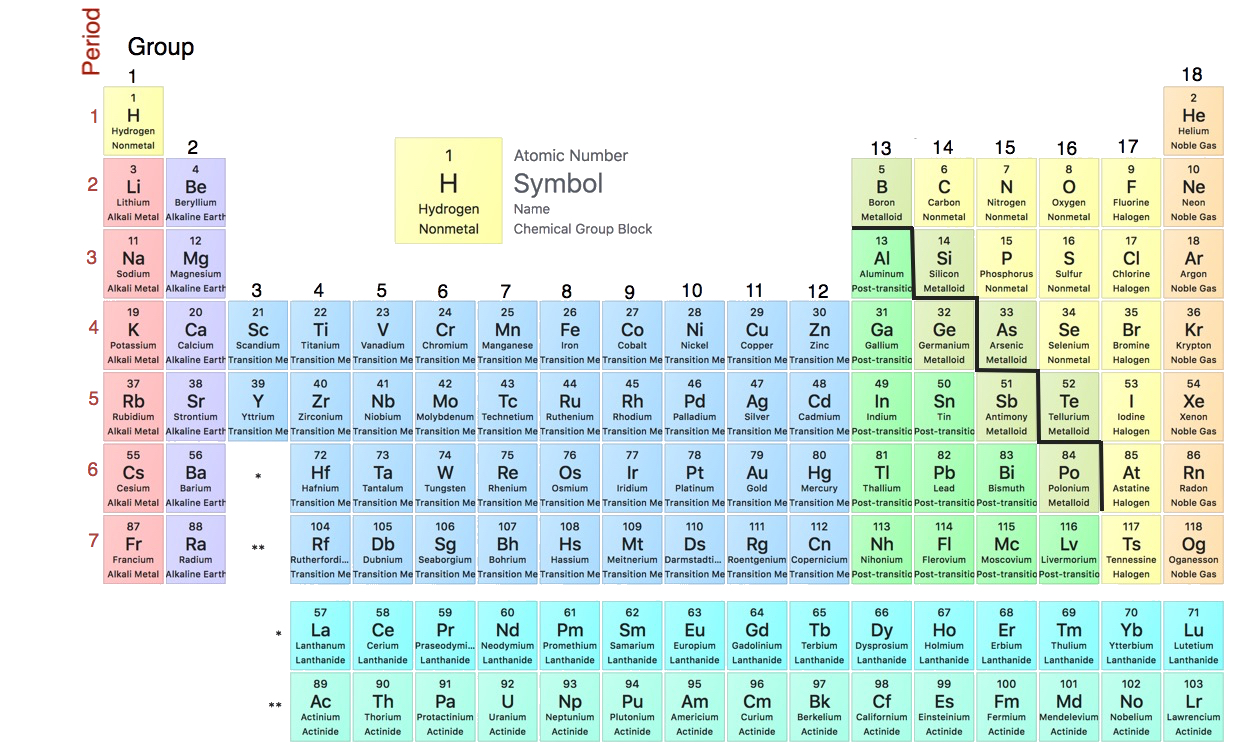
What is eighteen?
What is made of atoms?
Everything in the world is made out of atoms.
Name the charges of the 3 building blocks
Protons: positive charge, Neutrons: No/ neutral charge, Electrons: negative charge
These are the horizontal rows in the periodic table.
What is periods?
How do you covalent bond form?
Two atoms share electrons.
Phosphorus falls into this category.

What is nonmetal?
What is an element?
An element is a substance made from entirely one type of atom.
What is the nucleus of an atom?
the small, dense region consisting of protons and neutrons at the center of an atom
The first column of elements is this type of metal.
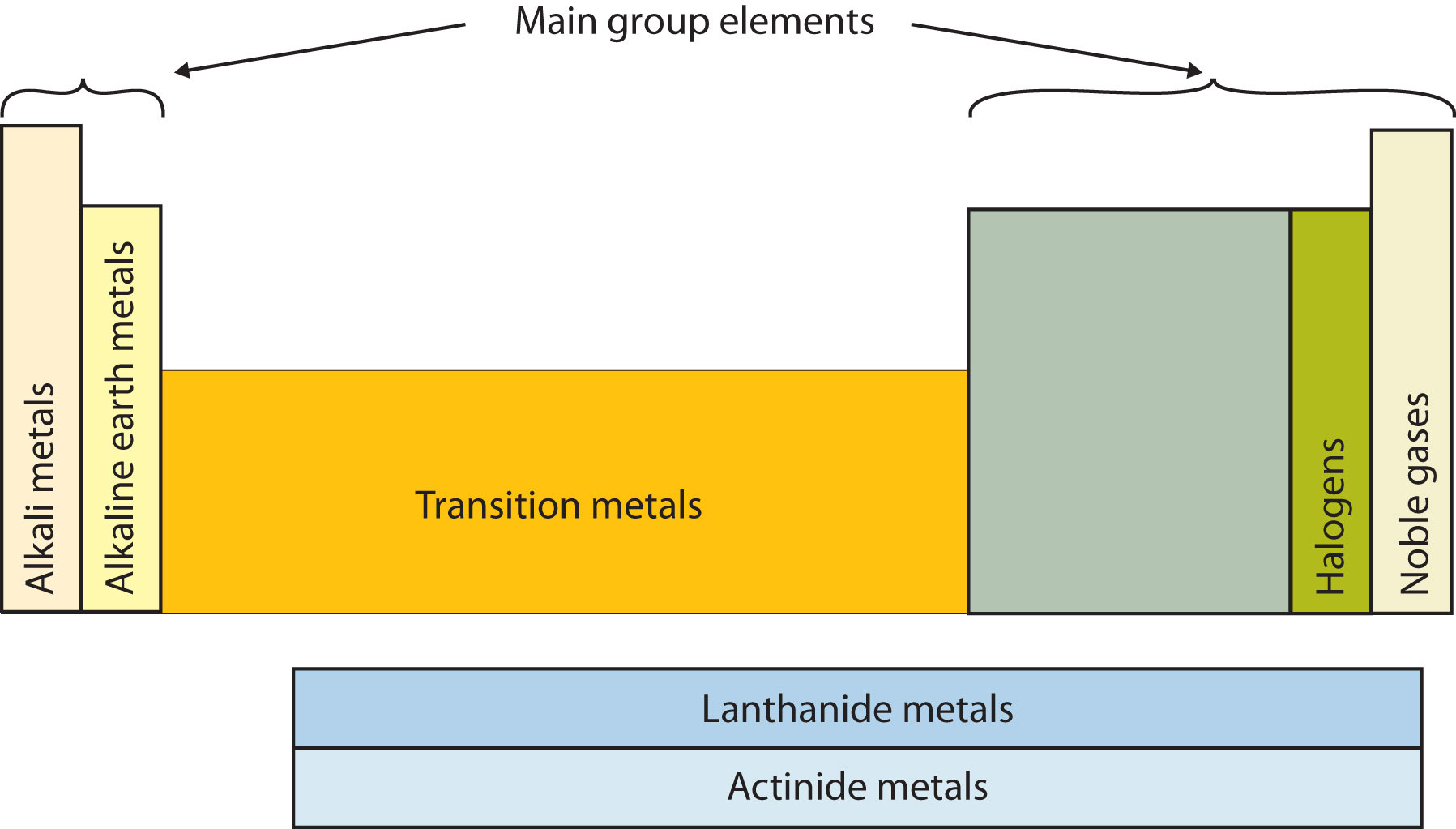
What is alkali?
What type of bond is H and F?
Ionic Bond
Estimate How many elements are there?
There are over 100 different types of elements.
True/false electrons are in the nucleus of an atom
False, they are on the electron cloud
Elements in the same group have the same _________ properties.
What is chemical?
What are one of the three ways the outer electrons of atoms can form?
2. Electron can be shared between atoms.
3. Electrons can be shared with all atoms in a material.
Elements can be broken down using chemical reactions, True or false?
False, because they are the simplest substances they can only be broken down with nuclear methods.
How do you find the mass of an atom?
The numbers of protons + the number of neutrons = atomic mass
This group of elements is farthest to the right.

What is noble gases?
What is the name and bond type of NaCl?
Sodium Chloride and Ionic Bond