Heat is a ____, which means the only thing that matters in energy transfer is the start and finish.
What is a state function?
The way that the equilibrium shifts when the temperature is decreased in this reaction:
N2(g)+3H2(g)⇌2NH3(g)
ΔH = -92.4 kJ/mol
Additional information about this particular reaction is present in the 300-point question description.
What is "shifting right" OR "shifting towards the formation of NH3"?
(Explanation: Decreasing the temperature favors the exothermic reaction, in this case the forward reaction.)
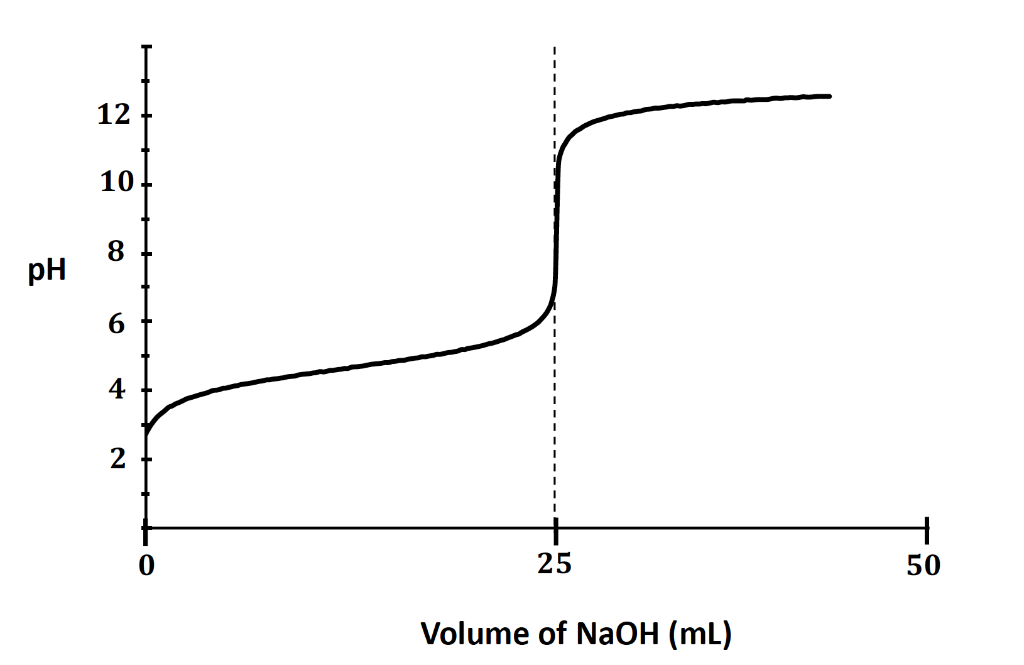
A titration between a ___ acid and a ___ base has an equivalence point above a pH of 7.
What is weak, strong?
The oxidation number of chromium in the compound H2Cr2O7.
What is +6?
The greatest fighter of all time.
Who is Tralalero Tralala?
(NOTE: If they said "Who is Bombardiro Crocodilo?", 100 points will be TAKEN AWAY from them. If they say anything else then no penalty will be issued.)
The total entropy change of this reaction in J/(molK) at 298 K:
2H2(g) + O2(g) -> 2H2O(l)
DeltaS values to know:
H2: 130.7 J/(molK)
O2: 205.1 J/(molK)
H2O: 70.0 J/(molK)
What is -326.5?
Given the reaction:
A(aq) + B(aq) -> 2C(aq)
The reaction quotient (Qrxn) where [A] = 0.20 M, [B] = 0.50 M, and [C] = 0.60 M.
What is 3.6?
The ratio of [NO2-]/[HNO2] in a buffer with a pH of 4.
pKa of HNO2 = 3.35
What is 4.47?
The species that are being oxidized and reduced in this reaction:
2NaCl(aq) + S(aq) -> ?
(This reaction may or may not be realistic.)
What is Cl- (oxidized) and S (reduced)?
The number of sigma skibidi rizz bonds that THREE O2 molecules have.
What is 3?
1. The Gibbs free energy change, in J/mol, of a chemical reaction where the temperature is 273.15ºC, the entropy change is 60 J/mol K, and the enthalpy change is -80 kJ/mol.
2. The thermodynamic favorability status of this reaction.
1. What is -112,778?
2. What is thermodynamically favorable?
The [NH3] at equilibrium after extra N2 is added so that [N2]=0.500M. Additional information is below.
The reaction N2(g)+3H2(g)⇌2NH3(g) is called the Haber process, with a Kc of 0.058 at 500ºC. At equilibrium: [N2]=0.418 M, [H2]=0.554 M, [NH3]=0.064 M.
What is 0.0687M?
The 7 strong acids.
What are HCl, HBr, HI, HClO4, HClO3, H2SO4, and HNO3?
1. The Eºcell for this voltaic cell.
2. The new Ecell when the [Zn2+] is 3M and the temperature is 50ºC.
E values to know:
Zn2+ + 2e- -> Zn: -0.76 V
Cu2+ + 2e- -> Cu: +0.34 V
1. What is 1.10 V?
2. What is 1.08 V?
The new element that Tony Stark created in "Iron Man".
What is "Badassium"?
(nabeel made this question)
The enthalpy change of this combustion reaction in J/mol:
C2H5OH + 3O2 -> 2CO2 + 3H2O
Use this table of bond energies for reference.
What is -1,245,000?
The Ka2 of carbonic acid (H2CO3) if:
Ka₁ = 4.3 × 10⁻⁷
In a 0.100 M solution, the concentration of carbonate ion [CO32-] is 9.9 × 10-8 M at equilibrium.
What is 4.7 x 10-11?
To get all of the points you need to answer all of these questions:
1. The pKa of the acid.
2. The pH of the equivalence point.
3. The pH range where [HA] > [A-].
4. The optimum buffering range of the acid.
1. What is 4.1? (answers close to 4.1 are fine)
2. What is 8? (answers close to 8 are fine)
3. What is 2.9-4.1? (answers close to this are fine)
4. What is 2.9-5? (answers close to this are fine)
The balanced form of this redox reaction in an acidic solution:
MnO4- + H2S -> Mn2+ + S
What is 2MnO4- + 6H+ + 5H2S -> 2Mn2+ + 5S + 8H2O?
Out of oxygen and nitrogen, which has the higher first ionization energy, and why?
Nitrogen, since its 2p3 subshell is more stable than oxygen's 2p4, even though it breaks the normal periodic table trend.
1. The enthalpy change of this reaction in J/molrxn: 2BiCl3(s) -> 2Bi(g) + 6Cl(g)
DeltaH values to know:
Bi(s) + (3/2)Cl2(g) -> BiCl3(s): -379.1 kJ/molrxn
Bi(s) -> Bi(g): +207.1 kJ/molrxn
2Cl(g) -> Cl2(g): -242.3 kJ/molrxn
2. This exact reaction commences in a huge ahh calorimeter filled with 8,884 grams of water at 60ºC. The reactants are placed inside and they produce 26.25 grams of Cl(g). Assuming 52,875 J of heat are lost to the surrounding atmosphere, calculate the new temperature of the water in Kelvin. (SHC of water: 4.18 J/(gK))
1. What is +1,899,300?
2. What is 325.41?
The molar solubility of Silver Sulfide in a mixture of 15.0 mL of 0.70 M Sodium Sulfide and 20.0 mL of 0.55 M Silver Nitrate AFTER the two solutions react with each other and form a precipitate.
Assume volumes are additive.
Ksp of Ag₂S= 6.31 x 10-50
What is 3.32 x 10-25 M?
The pH of a 20.0 mL, 0.50 M solution of formic acid (HCOOH):
1. Originally
2. After 12.0 mL of 0.30 M NaOH is added (assume volumes are additive)
The Ka of formic acid is 1.8 x 10-4. Assume you can use the x is small approximation.
1. What is 2.02?
2. What is 3.49? (The NaOH creates a buffer and turns 0.0036 moles of formic acid into its conjugate, so you use the buffer equation to solve.)
1. The standard Gibbs free energy change of an electrolytic cell where zinc is being plated onto a copper electrode, in kJ/molrxn.
2. The time, in hours, it takes for a 2.6 amp battery to help plate a copper electrode with 5.8 grams of zinc.
E values to know:
Zn2+ + 2e- -> Zn: -0.76 V
Cu2+ + 2e- -> Cu: +0.34 V
(no diagram this time haha shoulda thought about that when you picked 500)
1. What is 212.3?
2. What is 1.83?
What else is massive.
What is the low taper fade?