Define which partial charges are assigned to which atoms in H2O.
H is δ+ & O is δ-
What is this?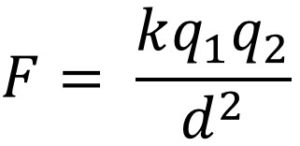
Coulomb's Law
What are ionic solids made out of?
metal cation and nonmetal/polyatomic anion
How many valence electrons does Boron have?
6
How many e- bonds and lone pairs are in a trigonal planar shape?
3 e- bonds & 0 lone pairs
How does electronegativity change going across a row and down a group on the periodic table?
increases when going left to right, decreases when going top to bottom
What factors affect bond length?
bond order & atomic radius
When can ionic compounds conduct electricity and why?
when molten into a liquid or dissolved into a solution; ions are free to move around and carry an electric current
Which elements can only have a total of 2 shared valence electrons?
Hydrogen & Helium
How many lone pairs does PCl3 have?
1
What determines polarity in a covalent bond?
the difference in electronegativity between the atoms
Define lattice energy.
the energy required to separate the ions in the ionic crystal lattice into individual ions
How should ionic compounds be drawn when dissolved in water?
H atoms faced toward anions , O atoms faced toward cations
What is the octet rule?
atoms tend to have 8 valence electrons/a full outer shell
Is NCl3 a polar or nonpolar molecule?
polar
How are metallic solids held together?
free moving / delocalized “sea” of valence e-
What is the relationship between bond length and bond strength?
inversely proportional
Why are ionic compounds brittle?
when force is applied, ions shift and experience repulsion forces, which breaks the crystal solid
How do you find the formal charge of an atom?
val e- - e- bonds - e- in lone pairs
What is the hybridization and bond angle of CH4?
sp3, 109.5˚
List the diatomic atoms.
H2, N2, O2, F2, Cl2, Br2, I2
Define the type and amount of bond types that each bond order has.
first: 1 σ; second: 1 σ, 1 π; third: 1 σ, 2 π
What are the general rules when drawing ionic solids? (list at least 3)
cations are usually smaller; anions are usually larger; use the correct cation:anion ratio; have a legend to identify the cation and anion; maximize attractive force and minimize repulsive force
What are resonance structures?
a set of two or more Lewis structures that can be used to describe a molecule or ion
In a BF3 molecule, what is the angle around Boron?
120˚