This formula represents one of the most important Laws for AP Chemistry
F = (q_1q_2 )/d^2
What is Coulomb's Law?
The greater the electronegativity difference the more ____ the bond becomes.
What is polar?
All molecules contain these types of forces.
What are London Dispersion Forces?
Gas pressure is caused by
What is the number of collisions?
Wavelength and frequency have this kind of mathematical relationship.
What is inverse?
Density is equal to ...
What is Mass / Volume?
Elements in the same ____ have similar properties.
What is group?
When a molecule is asymmetrical, it is this kind of molecule.
What is polar covalent?
High boiling and melting points and low vapor pressure and volatility means ____ IMFs.
What is large?
If you double the number of moles, the gas pressure will...
What is double (increase)?
When light has a long wavelength, it has ___ energy.
What is low?
During phase changes and chemical reactions, mass is...
What is conserved?
When reading a PES graph, the larger the binding energy means...
What is the electrons are closer to the nucleus?
Lattice energy is the energy required to break an ionic bond. This energy will increase when either of these TWO conditions occur.
What are ion's charge increases and ionic radii decreases?
What is the farthest?
The more molar mass a gas has, the _____ it moves.
What is slower?
Beer's Law is the measure of this vs this.
What is absorbance vs concentration?
During a distillation process of a solution, the mixture is separated based on...
What is boiling points?
Cations are smaller than their neutral atoms because...
What is valence electrons are removed from the farthest orbital from the nucleus and the nucleus will then pull the remaining electrons closer.
A triple bond contains ___ sigma bond, ___ pi bonds, and has __ hybrid orbitals.
The larger the molecule, the ____ polarizable and the ____ the London Dispersion Forces.
What is the more and stronger?
Temperature is a measure of the amount of this in a graph.
What is kinetic energy?
What is the Photoelectric Effect?
The percent composition by mass for any compound _____ change if it is a pure sample.
What is does not?
When an electron is in a higher energy level (farthest from the nucleus), it has ____ Coulombic attraction and is _____ to remove.
What is less and easier?
This molecule below has ____ molecular geometry, ____ bond angle, ____ hybridization, ___ sigma bonds, and ___ pi bonds.
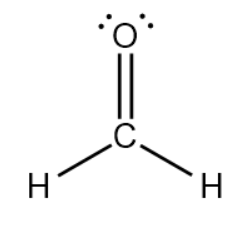
What are trigonal planar, 120o, sp2, 3, and 1?
Covalent Network solids are held together by this and not this.
What is covalent bonds and not IMFs.
Real gases behave most ideally at this and this.
What are high temperatures and low pressures?
This type of electromagnetic radiation has the highest amount of energy.
What is gamma rays?
These are the seven diatomic elements.
What are H2 O2 N2 Cl2 Br2 I2 F2 ?