This converting factor is used to convert values between grams and moles...
What is "Molar Mass"?
All metals atoms on the surface of an alloy do this thing in order to create a "Sea of Electrons"...
What is "lose or release their valence electrons"?
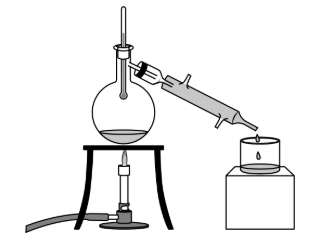
The image above depicts this specific separation technique...
What is "distillation"?
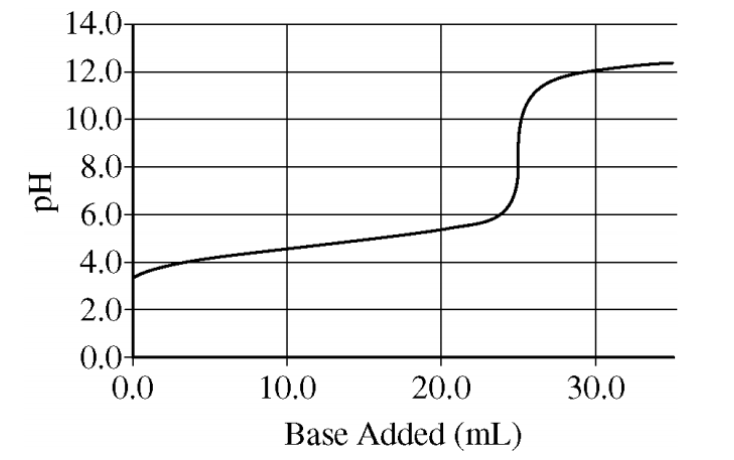
According to the graph, this is the volume of base that was needed to reach the equivalence point in a titration.
What is "25 mL"?
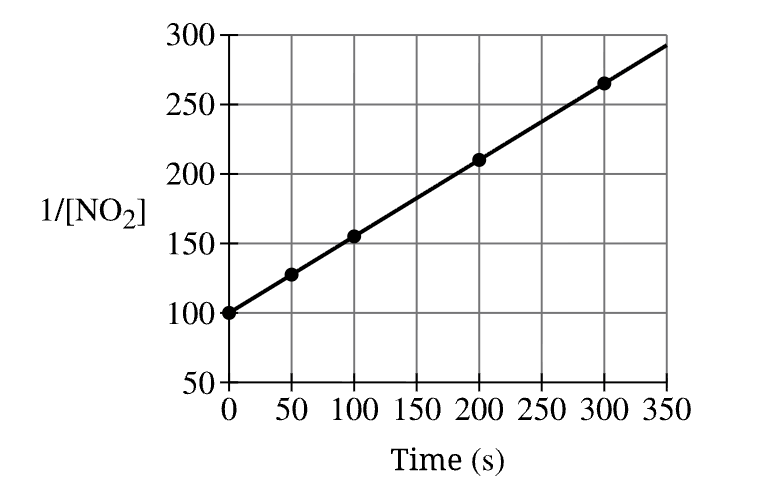
The graph identifies NO2 to have this reaction order...
What is "second order"?
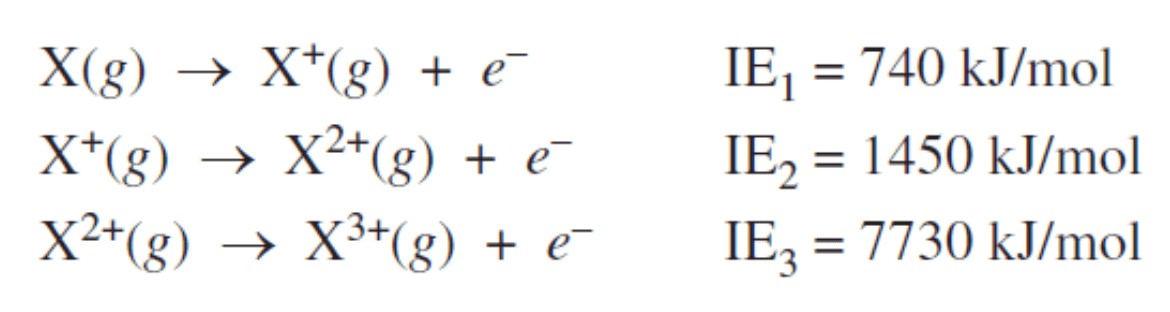
Based on the given data, element X has this many valence electrons...
What is "two valence electrons"?
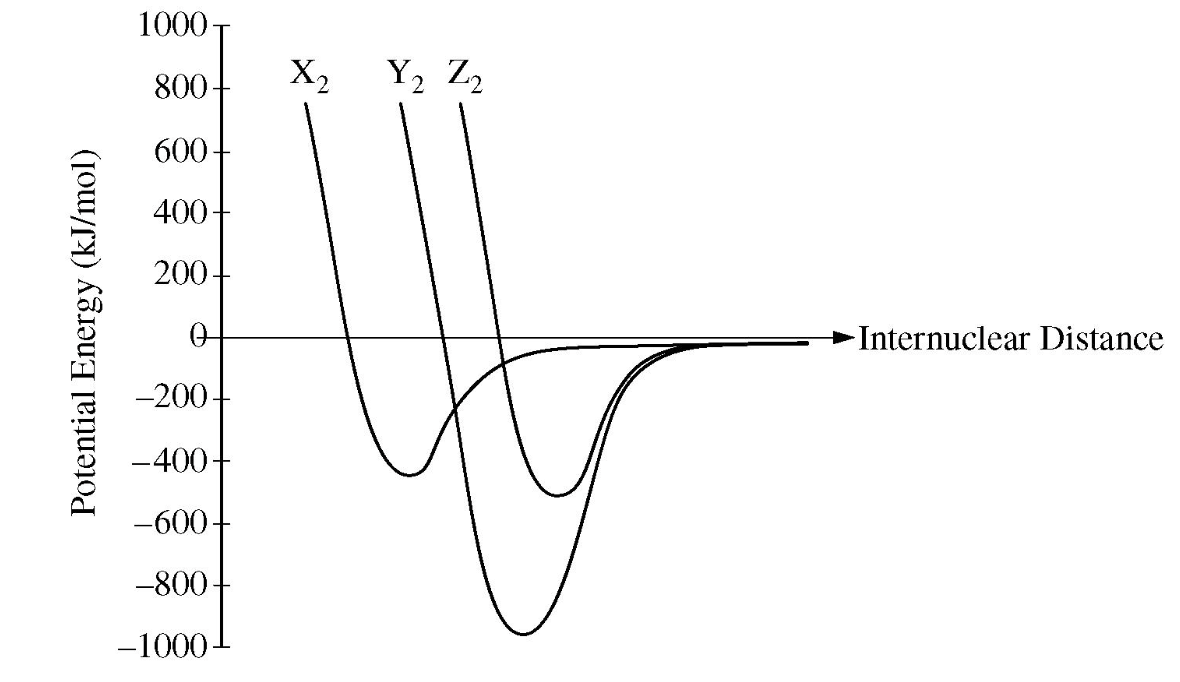
Based on the graph, Z2 likely has this type of bond...
What is a "double covalent bond"?
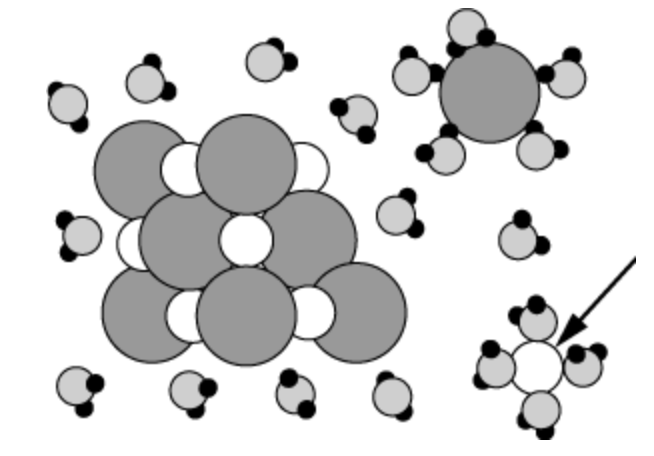
The arrow is pointing at this type of ion...
What is a "cation"?
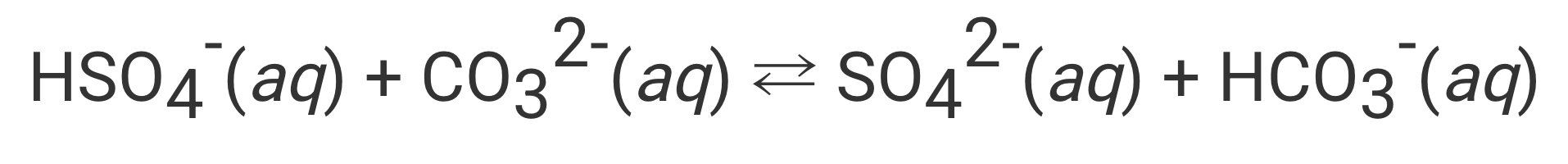 ________ is considered a conjugate base in the above reaction.
________ is considered a conjugate base in the above reaction.
What is "SO42-"?
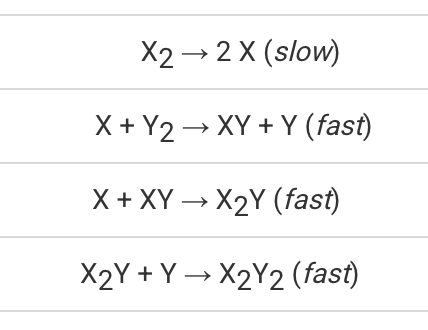
This is the rate law based on the given reaction mechanism...
What is "Rate=k[X2]"?
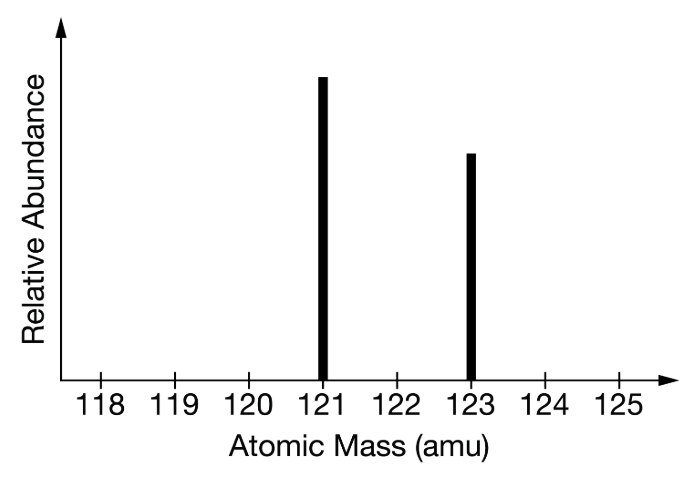
This mass spectrum graph represents this element from the Periodic Table...
What is "Antimony" or "Sb"?
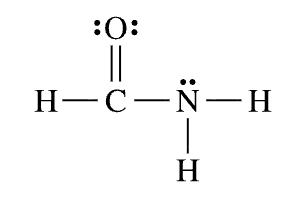
The above molecule has this many nonpolar bonds...
What is "one nonpolar bond"?
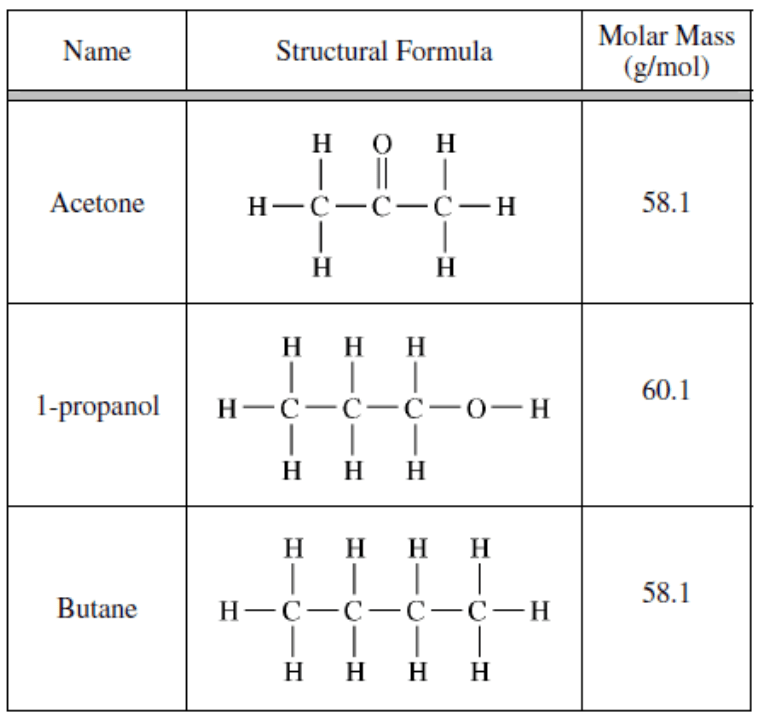
This molecule from the above list is most likely to evaporate first at room temperature...
What is "butane"?
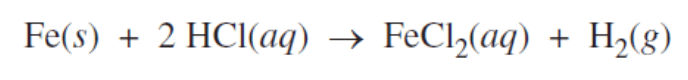
This substance is being reduced in the above chemical reaction.
What is "hydrogen"?
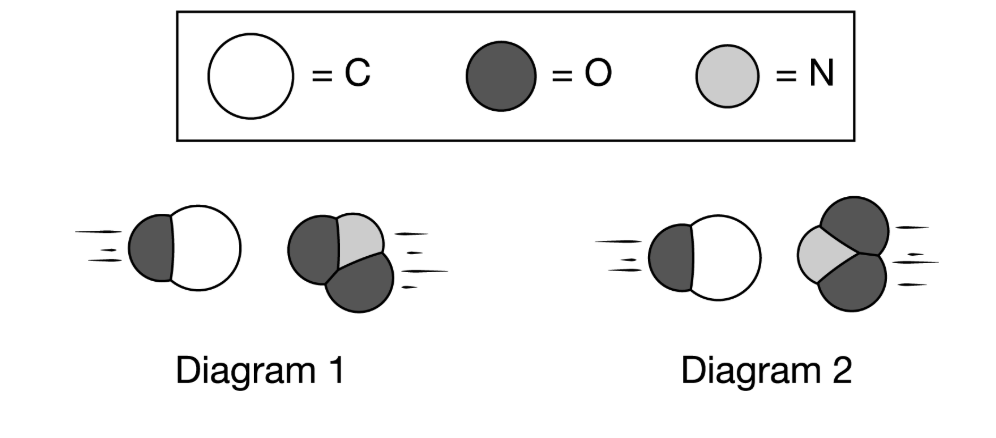
Diagram ___ accurately represents an effective collision that would result in the formation of CO2.
What is "1"?
The ground state electron configuration of Scandium, Sc is...
What is "1s22s22p63s23p64s23d1"?
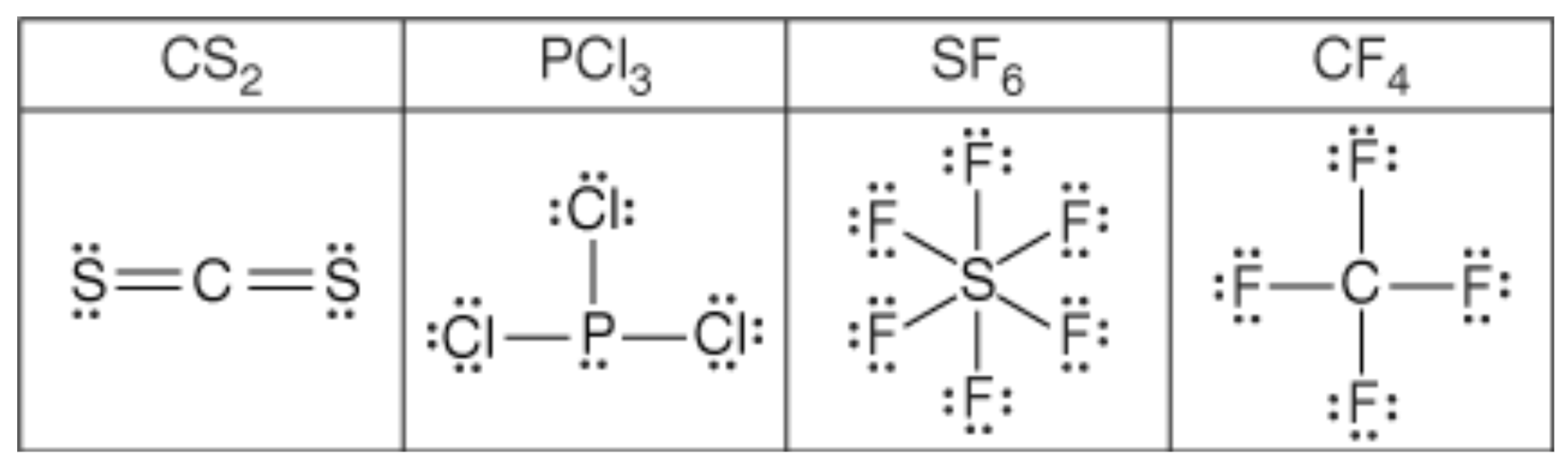 Out of the given options, _________ is the most polar molecule.
Out of the given options, _________ is the most polar molecule.
What is "PCl3"?
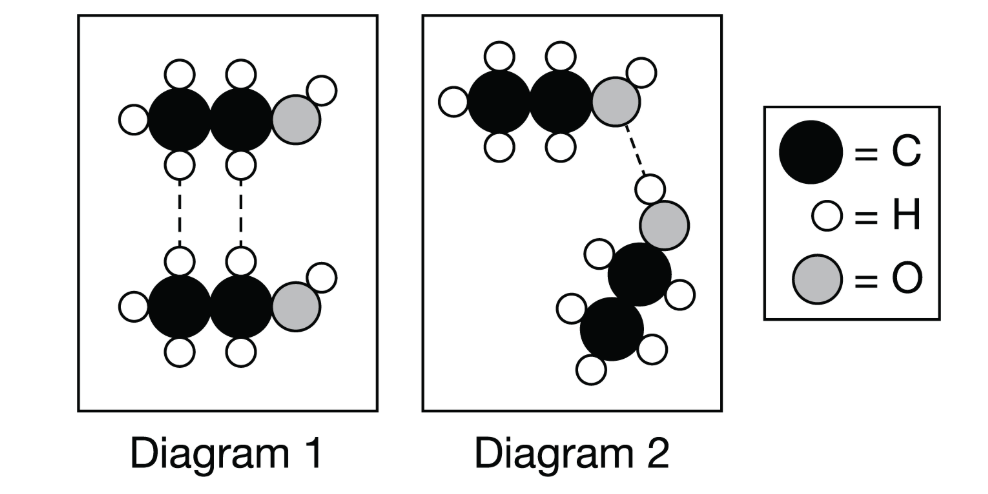
________ _________ is being represented with the dashed lines in Diagram 2.
What is "hydrogen bonding"?
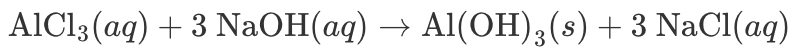
____ and ____ would be considered spectator ions in the complete ionic equation for the above reaction.
What are "Na+ and Cl-"?
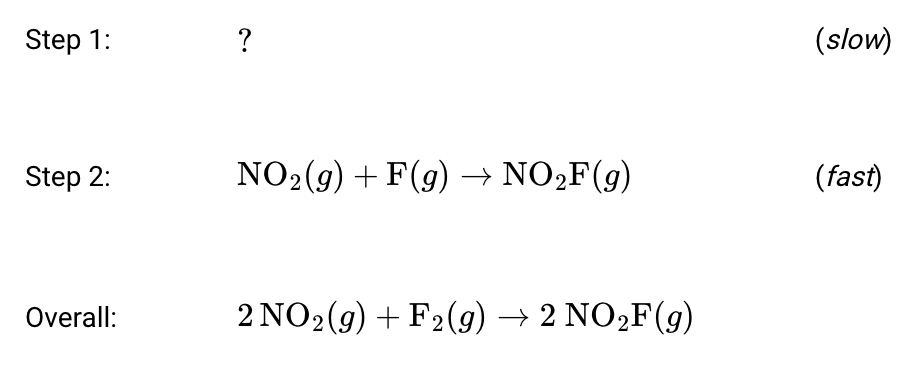
Based on the provide reaction mechanism, this substance is likely a product in Step: 1...
What is "F(g)"?
Sodium metal is more reactive in water than magnesium metal because...
What is "Sodium has a smaller first ionization energy than magnesium"?
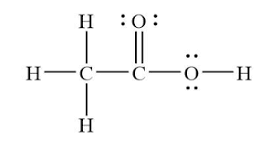
This is the bond angle for the oxygen-carbon-oxygen in CH3COOH...
What is "120o"?
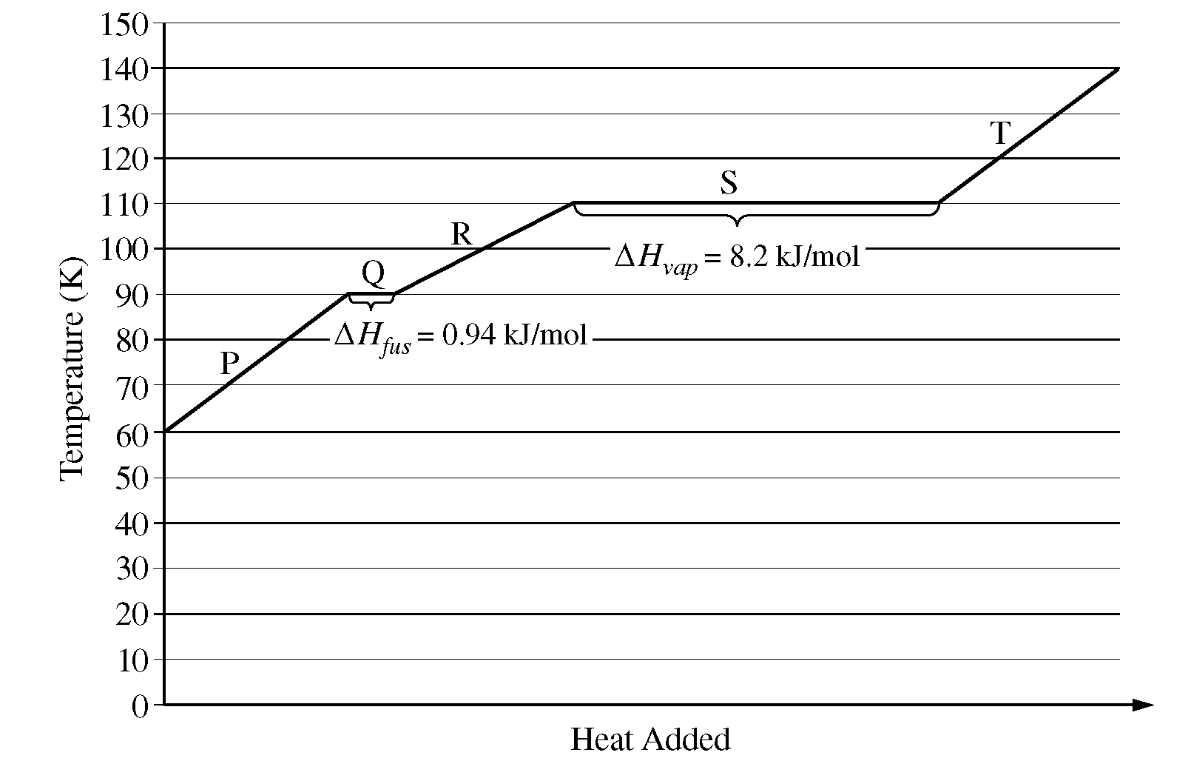
This is the reason why 'S' has a greater ΔH than 'Q' on the graph above...
What is "overcoming all or most intermolecular attractions"?
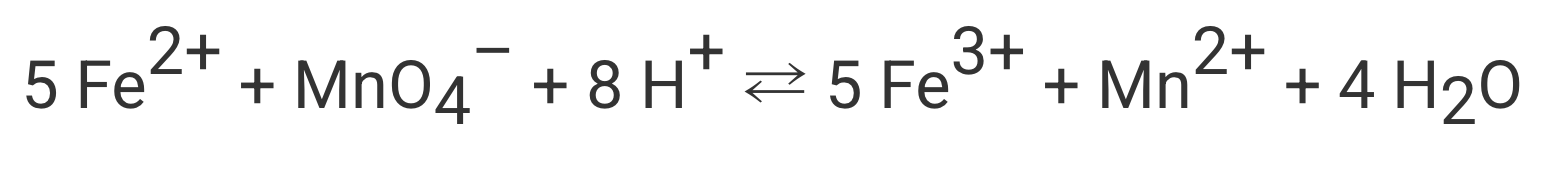
This many moles of MnO4- would be required to completely react with 10.0 moles of Fe2+...
What is "2.0 moles"?
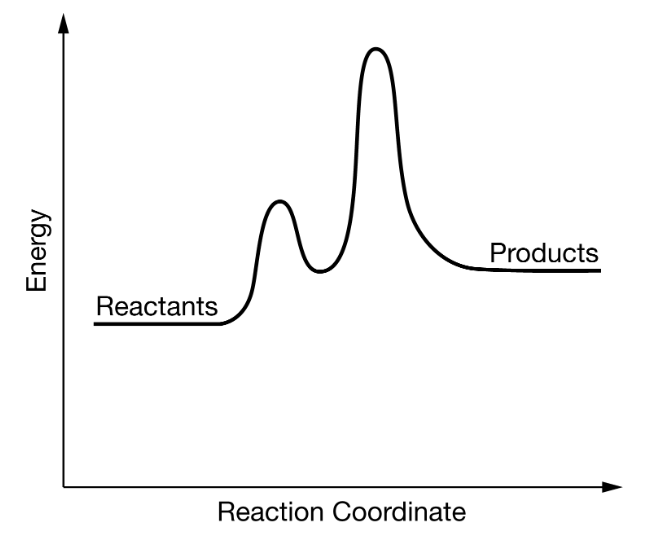
This step is considered the 'fast' step in the above reaction energy profile graph.
What is "Step 1"?