What element does this photoelectron spectrum represent? Assume that the element is in its ground state.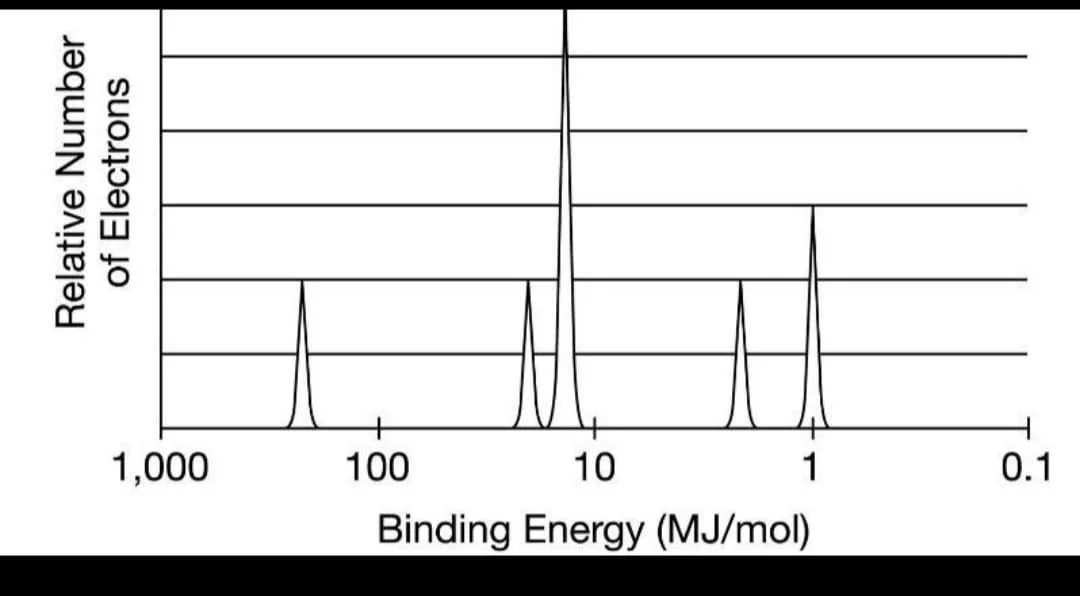
Phosphorus
What type of alloy would form from the mixture of titanium and chromium?
Substitutional
What is the strongest intermolecular force contained in acetone?
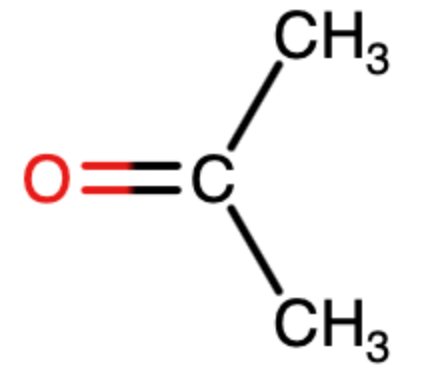
Dipole-Dipole Interactions
1.0 L of a 1.5 M solution is boiled to decrease the volume to 500 mL. What's the concentration of the new solution?
3.0 M
What is the mole ratio to convert from sulfuric acid to water?
H2SO4 + NaOH --> Na2SO4 + H2O
(2 mol H_2O)/(1 molH_2SO_4)
What are the two requirements for a collision to react, according to collision theory?
Sufficient energy and proper orientation
What is the molar mass of ZnSO4 • 7H2O?
287.55 g/mol
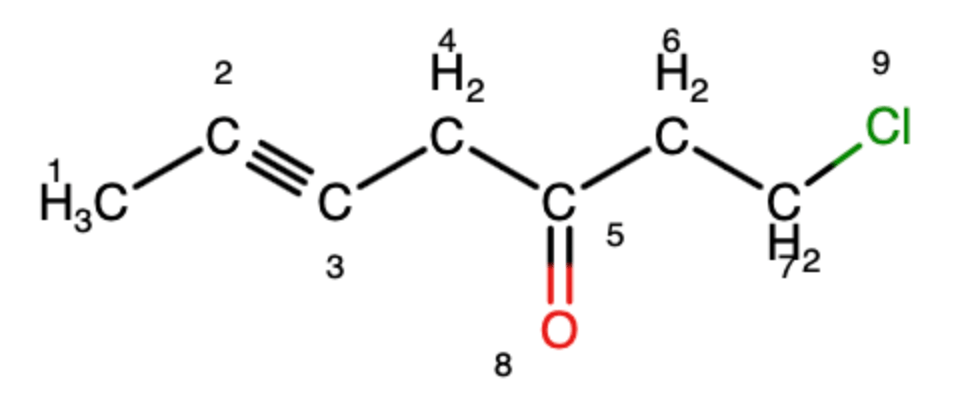
What is the hybridization of carbon 2?
Name 2 of the postulates that we assume are true for an ideal gas, according to the kinetic molecular theory.
1. Gas particles are in constant random motion.
2. IMFs between gas particles can be assumed to be negligible.
3. Volume of individual gas particles can be assumed to be negligible.
4. The average kinetic energy is proportional to the Kelvin temperature.
The absorbance of a sample is measured. How would the absorbance change if a 5.0 cm cuvette was used instead of a 1.0 cm cuvette?
Absorbance would increase (by a factor of 5)
Is iron being oxidized or reduced?
Fe3+ --> Fe
Reduced
What is the order of a reaction with the following graphs?
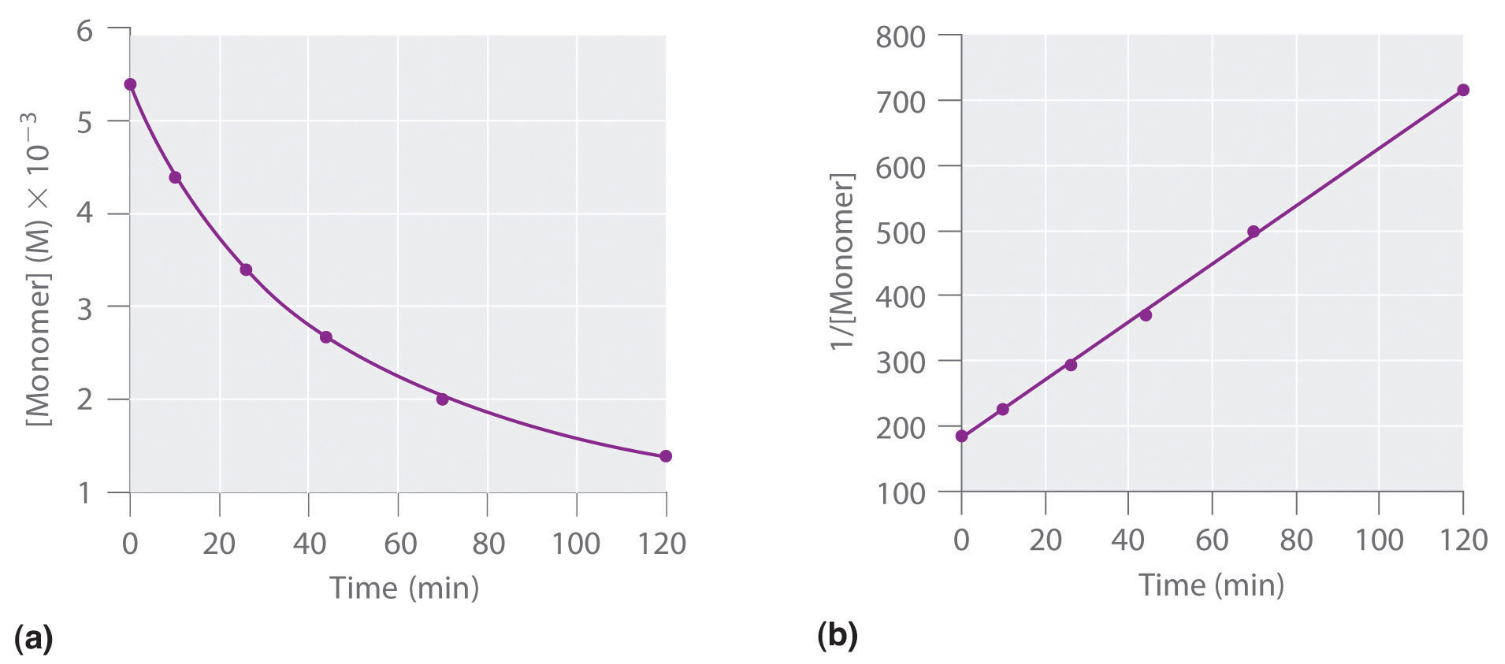
Second Order
Which of the following elements will have the smallest electronegativity? Justify your answer.
O, S, Se
Se because it has the largest number of energy levels, causing the force of attraction on external electrons to be very small, due to the inverse relationship according to Coulomb's Law.
What is the molecular geometry and bond angle of Ammonium?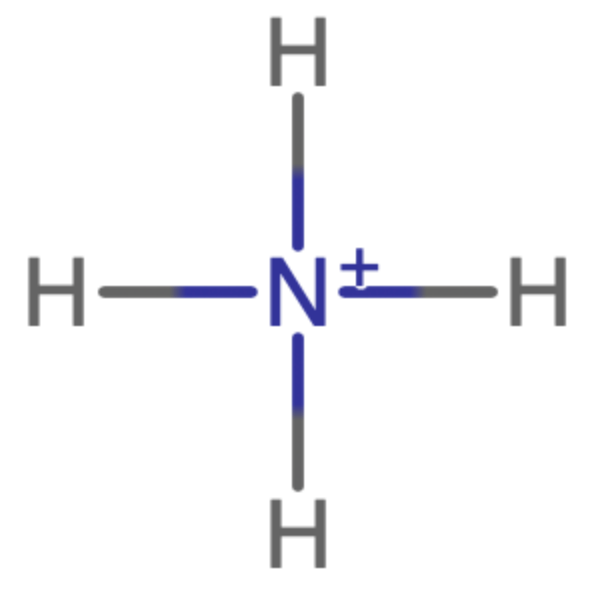
Tetrahedral - 109.5˚
An ideal gas is compressed from V to 1/3•V at a constant temperature. What is the value of the final pressure in terms of the initial pressure, P?
3•P
What type of spectroscopy would need to be used to study molecular vibrations?
Infrared
By convention, what substance is held in the buret of a titration experiment?
Titrant
Calculate the rate law using the initial rate of reaction, based on the concentration of NO and Cl2.
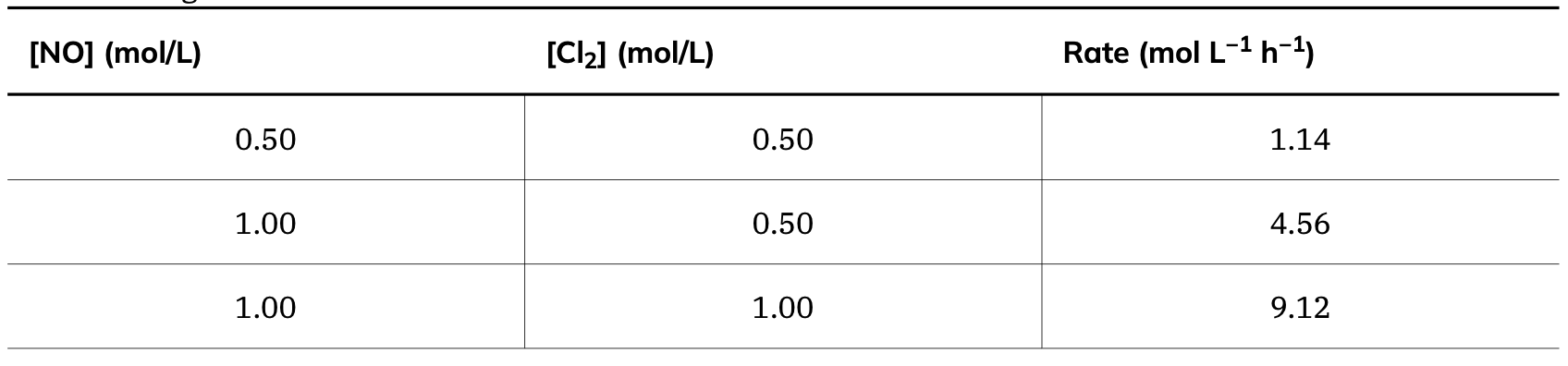
Rate = k[NO]2[Cl2]
What is the noble gas electron configuration of Fe2+?
[Ar]4s23d6 --> [Ar]3d6
What is the bond length of the molecule associated with the potential energy curve?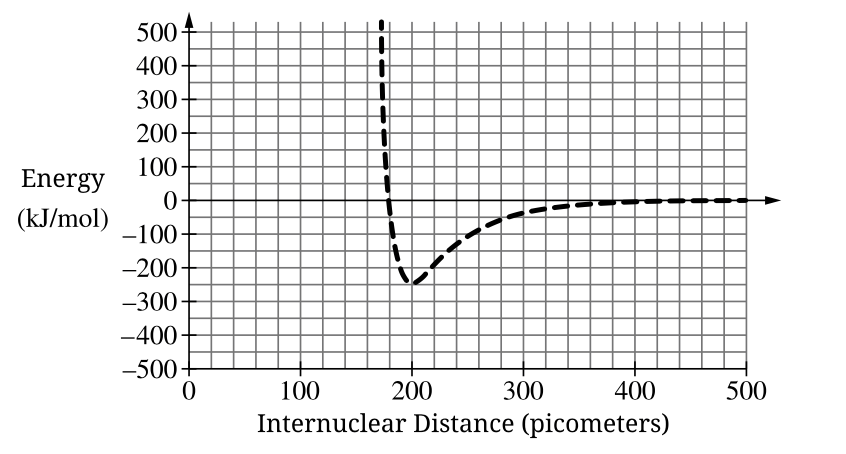
200 pm
A researcher finds that a gas at low temperature has a lower volume than predicted by the ideal gas law. Explain this phenomenon.
Due to low temperature, the gas molecules are moving much slower, giving particles time to exert attractive intermolecular forces on one another. This causes molecules to become closer together, decreasing the sample volume.
What type of intermolecular force is pictured?

Ion-dipole
Is the boiling of water a chemical or physical change? Explain your answer in terms of the forces that are being changed.
Physical change - the IMF's (hydrogen bonds) are breaking between water molecules instead of the bonds within the water molecules.
What are the units of the rate law constant for a 5th order reaction?
1/(M^4*s) or M^-4*s^-1
Which of the following has the largest ionization energy? Justify your answer relative to both other options.
P, N, F
F because...
• F>N due to higher number of protons, increasing charge which is directly proportional according to CL
• F>N due to fewer number of energy levels, decreasing distance, which is inversely proportional to CL
Which of the following Lewis Structures is the most valid? Justify your reasoning.
A.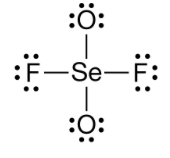
B.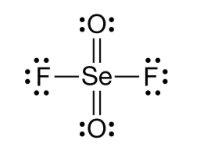
B - formal charges are overall closer to 0.
Would you predict decane (C10H22) or octane (C8H18) to have a higher vapor pressure?
Octane due to lower intermolecular forces. Both decane and octane only possess LDF's. Longer carbon chains are more polarizable, due to a higher number of electrons, causing decane to have higher IMF's and, therefore, a lower vapor pressure as molecules are held together tighter.
A carbon-oxygen double bond (such as those found in carbon dioxide) has a bond energy of 800 kJ/mol. What frequency would a photon need to be to have sufficient energy to break this bond?
2.00 x 1015 Hz
What is the limiting reagent when 8.0 g of sodium reacts with 8.0 g of chlorine?
2Na + Cl2 --> 2NaCl
What is the half life of the following rate law?
R=(1.21 x 10^(-4)yr^-1)[`C-14`]
5730 yr