This is the reason why MgO has a greater lattice energy than NaCl.
What is the ion charges of MgO are greater than the ion charges in NaCl.
This is number of valence electrons needed on a Lewis structure for the phophate ion.
What is 32?
The molecular geometry for H2O
What is bent
The bond angle(s) for a see-saw shape molecule.
What is 90 and 120, or less than 90 and less than 120
The type of alloy shown in the picture below.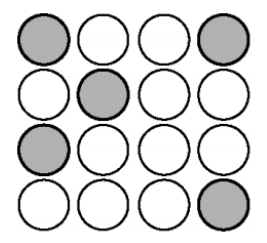
What is substitutional?
This is represented on the graph by the section labeled "x"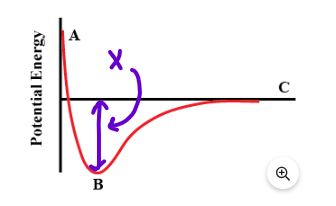
What is bond energy or bond strength
The number of electron domains & lone pairs around the central atom in IF4-.
What is 6 electron domains & 2 lone pairs.
The molecular geometry for BrF3.
T-Shaped
The bond angle for the molecule shown below.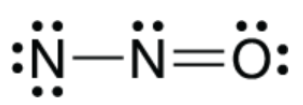
What is 120 or less than 120
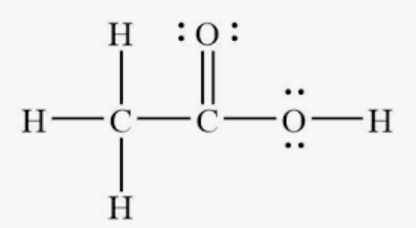
The hybridization of the orbitals around the carbon atoms in the highlighted section of the molecule shown below.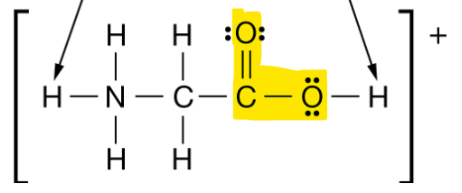
What is sp2
Of the following, the one with the greater lattice energy. Include the reason in your answer.
LiF, NaCl
What is LiF because the ions are closer together
Of the following, the molecule where the central atom has an expanded octet.
SO42-, ClF3, SO2, NCl3
What is ClF3
The molecular geometry for SO32-.
Trigonal Pyramid
The C-O-H bond angle for the section of the molecule highlighted below. 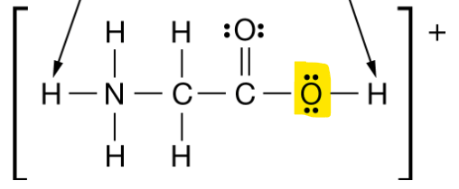
What is 109.5 or less than 109.5
This is the formal charge around the central atom in the molecule shown below: 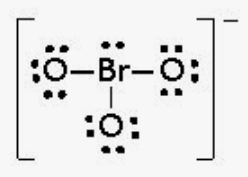
What is +2?
Of the bonds listed below, the one with the greatest ionic character.
O-F, N-F, P-F
What is P-F?
Of the following, the structure that will be represented by two or more resonance structures.
SO2, CO2, BF3, SO42-
What is SO2
Of the following, the structure with a square pyramid molecular structure.
PCl5, ClF5, XeF4
What is ClF5
Of the following, the one that is NONPOLAR.
H2O, NF3, NO3-, HCl
What is NO3-
Localized or delocalized pi bonds?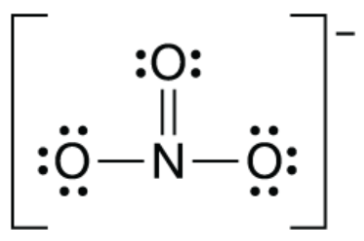
delocalized
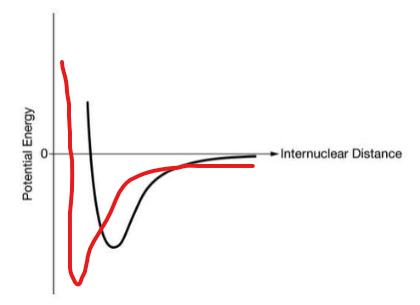
The graph below represents the potential energy vs distance between atoms in a Br2 molecule. Sketch a graph that would represent the PE vs distance between atoms in an O2 molecule. 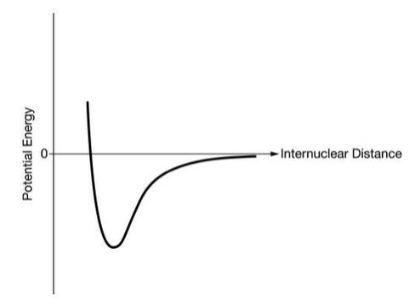
The structure for the molecule CH3COOH
The number of structures listed below that are NONPOLAR.
BF3, H2S, HF, PF5, CO2, SF6
Of the following molecules, the one with the SMALLEST bond angle.
CH4, NH3, NO2-, NO3-
What is NH3?
Describe the N-O bond length in nitrate ion.
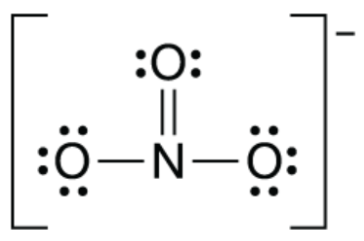
They are all the SAME length with a bond order of 1 1/3