A student has a 1g sample of each of the following: NaCl, KBr, and KCl. List the samples in order of increasing number of moles in the sample.
What is KBr < KCl < NaCl
What type of bonding occurs in HF?
What is a polar covalent bond
HF has a much smaller electron cloud than F2, but the boiling point of HF is 293 K versus 85 K for F2. Explain the discrepancy between their boiling points.
What is....HF has hydrogen bonding, a strong intermolecular force that takes significant amount of energy to overcome
A student mixes 20g of white KCl crystals in distilled water in a beaker. After the mixture was stirred, no crystals are visible and the solution is clear. After several days, the water evaporates and white crystals are found in the beaker. What experimental evidence would help the student confirm that a new compound has not been made and only a physical change occured?
the white crystals have a mass of 20g
When the chemical reaction 2 NO + O2 -> 2 NO2 is carried out under certain conditions, the rate of disappearance of NO is 5 x 10-5 M/s. What is the rate of disappearance of oxygen gas under the same conditions?
2.5 x 10-5 M/s
A student obtains a sample of a pure solid compound. In addition to Avogadro's number, what does the student need to know in order to calculate the number of molecules in the sample? ( 2 things)
What is the mass of the sample and molar mass
What is responsible for the lattice energy of MgCl2 being much greater than the lattice energy for NaCl?
what is the charge of the Mg cation being much larger than the charge from the sodium ion
Which of the following would have the highest vapor pressure and why? Methane, Pentane, Heptane, Octane
Methane because it has the weakest IMF, therefore the most methane can escape into the gas phase, causing a higher vapor pressure
Equal volumes of solutions of lead (II) nitrate and potassium bromide are combined to form lead (II) bromide as a yellow precipitate. Write the correct net ionic equation for this reaction.
Pb2+(aq) + 2Br- (aq) -> PbBr2 (s)
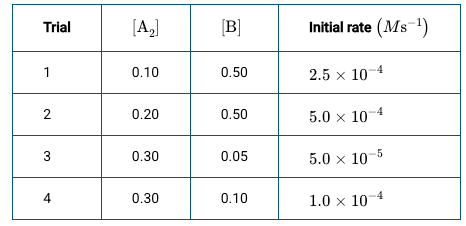
Using the data above, what would happen to the rate if the concentration of the reactants are doubled?
rate = k[A2][B], rate would quadruple
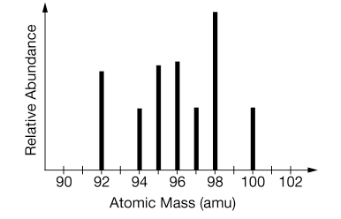 Identify the element from the mass spectrum
Identify the element from the mass spectrum
What is Molybdenum
What is the bond angle for NH3?
<109.5
A gas mixture at 273 K and 1 atm contains .01 mol hydrogen, .015 mol oxygen, and .025 mol nitrogen. Assuming ideal behavior, what is the approximate partial pressure of hydrogen in the mixture?
What is about 0.20 atm because hydrogen is about 20 percent of the gas mixture.
When C2H4 reacts with hydrogen gas, the compound C2H6 is produced. What type of reaction occurs, justify your answer.
oxidation-reduction because hydrogen is oxidized
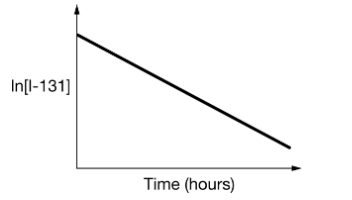
Draw a mock graph with labeled axis for the decay of I-131 that could be used to find the value of k. The value of k = 3.6 x 10-3 hours-1
What would the charge of the following element be? 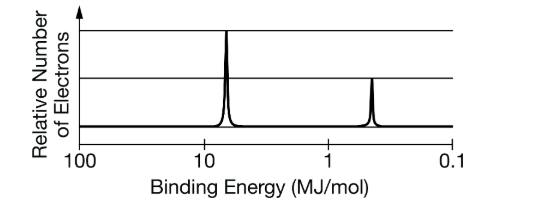
What is + 1
Draw the lewis diagram for the nitrate ion, include resonance

A sample of helium gas at 25 degrees and 1 atm is combined with a sample of neon gas at 25 degrees and 1 atm. The temperature is kept constant. What happens to average kinetic energy of the two gases when combined? Explain.
A 100 mL sample of 0.1 M MgCl2 and 100 mL sample of 0.2 M NaOH were combined and magnesium hydroxide precipitated. If the experiment is repeated using solutions of the same molarity, what change could be made in volume to double the amount of precipitate produced?
doubling the amount of both reactants would double the amount of products
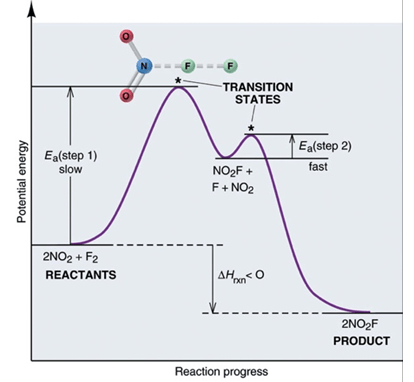
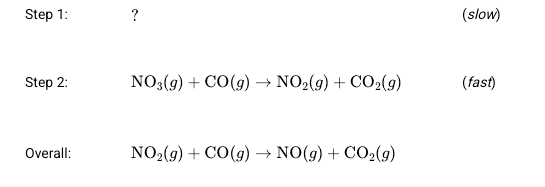 Using the reaction mechanism above. What is the chemical equation for step 1 AND the rate law for the overall reaction?
Using the reaction mechanism above. What is the chemical equation for step 1 AND the rate law for the overall reaction?
Rate = [NO2]2 , step 1 = 2 NO2 -> NO + NO3
Explain the difference in electronegativity between fluorine and chlorine
For SF4, what is the molecular shape, hybridization, and bond angle/s?
What is see-saw, sp3d, <90/120
A student uses spectrophotometry to determine the concentration of CoCl2 in a sample solution. The student made the standard curve below. What likely caused the error in the point plotted at 0.05 M? 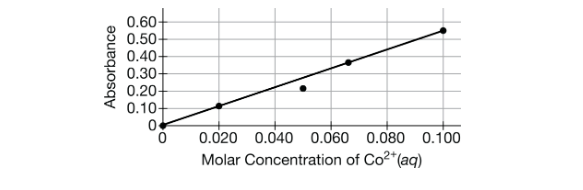
Likely some distilled water in the cuvette when they put the standard solution in. The diluted solution would have a lower absorbance.
Write the oxidation reduction reaction between aluminum and zinc and calculate the total number of electrons transferred.
6 electrons transferred. 2 Al (s) + 3 Zn2+ -> 2 Al3+ + 3 Zn (s)
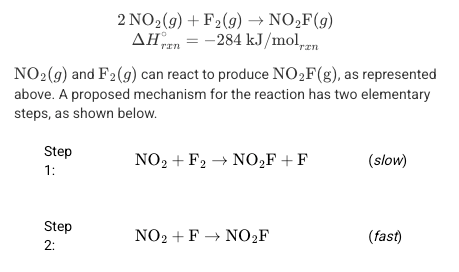
using the data above, draw a reaction energy diagram that shows the following details
1) the relative activation energies of the two elementary steps
2) the enthalpy change of the overall reaction