Every atom of the same element has the same number of these
What is protons
These two sub atomic particles contribute to mass
What are protons and neutrons
These are the valence electrons
What are the most outer electrons
The atomic number tells us this about each element
What is number of protons
This element has 26 protons
What is Iron
Hydrogen - 2
This is an example of what type of isotope notation
What is hyphen
This is where the electrons are located
What is the electron cloud
This is the number under each symbol with decimals (ex for H = 1.008)
What is average atomic mass
Where are the protons found in an atom?
Where is the nucleus
In two isotopes of the same element the atoms will have the same _______ but different ______
Same = protons
Different = neutrons
This is how many valence electrons does bromine (Br #35) have?
What is 7
This is the name of all the metals in the middle of the periodic table
What is transition metals
Holmium (Ho) has how many protons
What is 67
Gallium-70 has this many neutrons
What is 39
** DAILY DOUBLE **
Draw the Bohr model for Hydrogen, fluorine and aluminum
----
This group is the most reactive metals
What are the alkali metals (gp 1)
What are protons repel each other and protons attract electrons
What is nickel
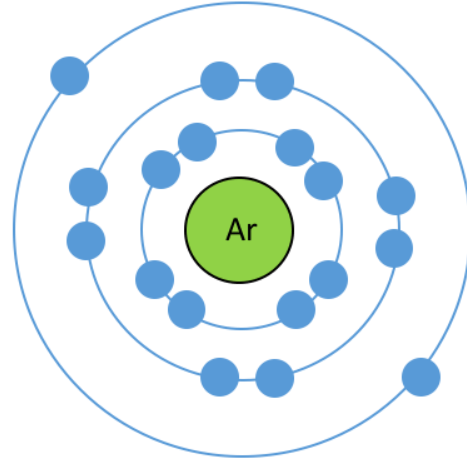
These are the TWO problems with this Bohr Model
What is there can only be 2 electrons in the first shell and incorrect valence electrons
This element has 4 valence electrons and 6 E levels
What is lead (Pb)