Salad is an example of this type of mixture

What is a heterogenous mixture?
The subatomic particle with a positive charge.
What is a proton?
The element used to fill balloons and make them float
:max_bytes(150000):strip_icc():focal(749x109:751x111)/macys-parade-110422-1-0aa5da72a3834324b311b4ea08859b9a.jpg)
What is helium?
The name of the type of subatomic particle indicated by the arrow in this image:

What is a neutron?
This type of diagram shows all of an element's electrons in shells. For example:

What is a Bohr diagram?
Sulfur is an example of this type of pure substance

What is an element?
The subatomic particle with a negative charge.
What is an electron?
The element used to make thin sheets of foil for cooking and baking

What is aluminum?
The name of the type of subatomic particle indicated by the arrow in this image:

What is a proton?
The name of the notation that indicates electron energy levels, sublevels and numbers of electrons for an element.
For example, 1s2 2s2 2p6 3s1
What is an electron configuration?
Water is an example of this kind of pure substance

What is a compound?
The two types of particles found in the nucleus
What are protons and neutrons?
The name of the element with the symbol Na
What is sodium?
The name of the type of subatomic particle indicated by the arrow in this image:

What is an electron?
The name of the notation that starts with a noble gas in square brackets and indicates electron energy levels, sublevels and numbers of electrons for any additional electrons.
For example, [Ar] 4s2 3d5
What is an abbreviated electron configuration or noble gas electron configuration?
Salt is an example of this type of pure substance

What is a compound?
The type of particle with negligible (almost zero) mass
What are electrons?
The name of the element with the symbol Au
What is gold?
The name of the location indicated by the bracket and arrow in this image:

What is the nucleus?
This type of diagram shows only valence electrons. For example:
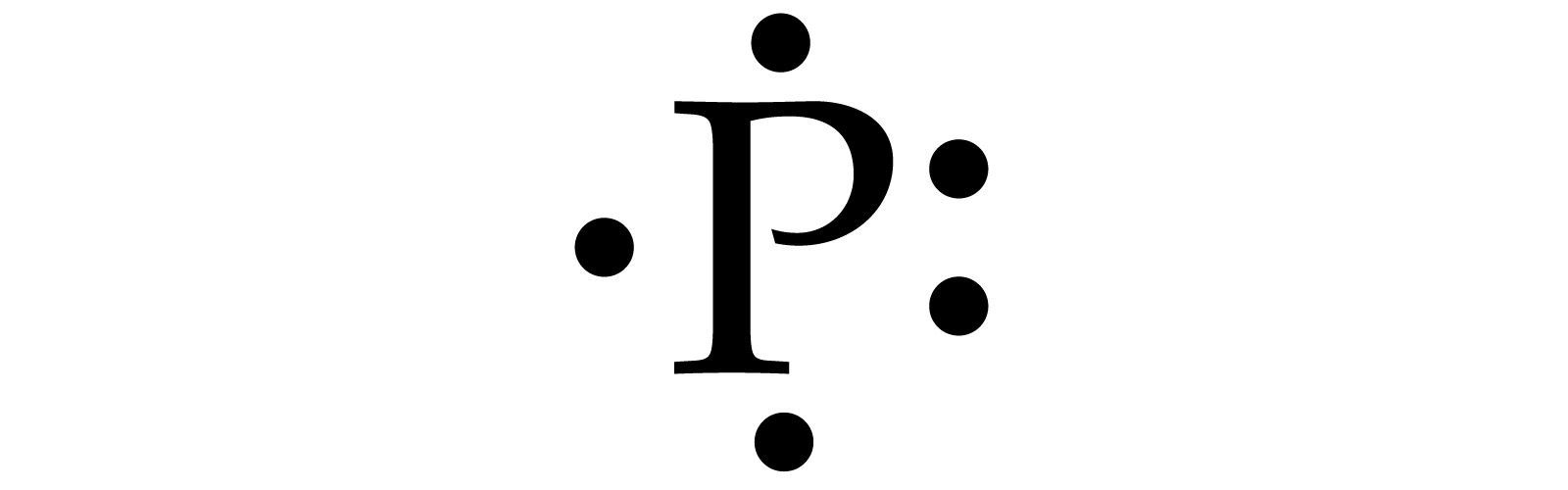
What is a Lewis diagram or Lewis dot structure?
Air is an example of this type of mixture
What is a homogenous mixture?
To calculate the atomic mass, you add the numbers of these types of particles
What are protons and neutrons?
The element that makes up 78% of air
What is nitrogen?
This type of atomic diagram shows only the element symbol and valence electrons. For example:
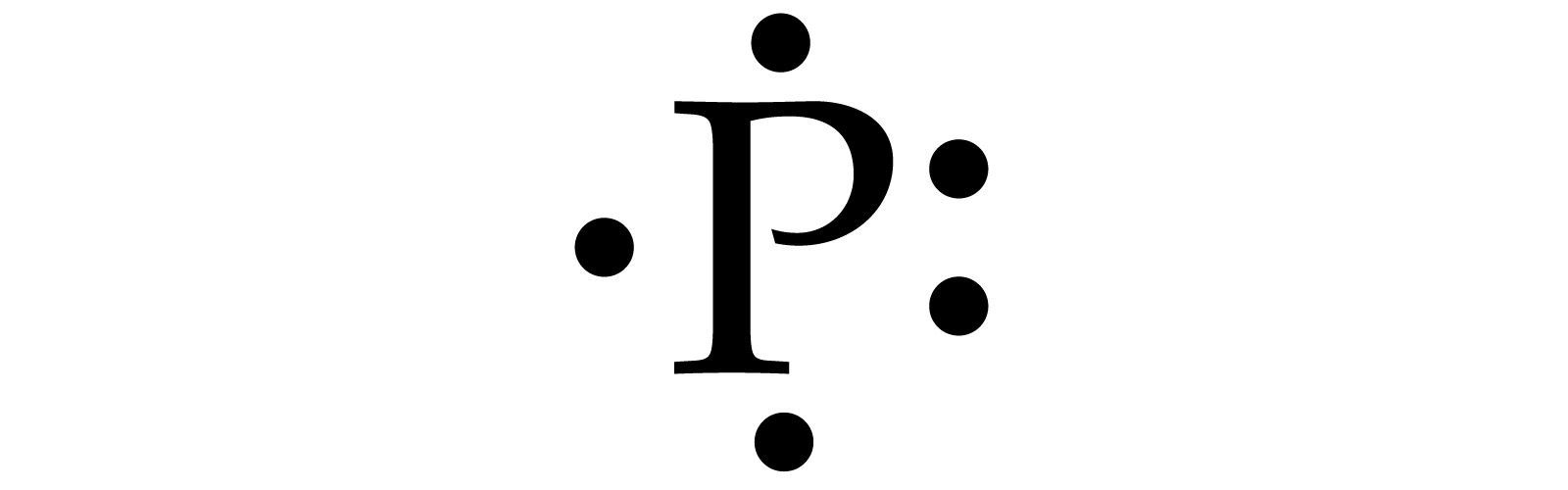
What is a Lewis diagram or Lewis dot structure?
The name of the type of drawing that shows how an atom's orbitals are occupied by electrons. For example:

What is an orbital diagram or orbital filling diagram?
Steel, brass and different types of gold (yellow, white, green and rose) are examples of this type of homogenous mixture

What is an alloy?
Isotopes have the same number of _____ but different numbers of _____
What are protons and neutrons?
The name of the element that is an exception to the rule that group 18 elements have 8 valence electrons
What is helium?
This type of atomic diagram shows all of an element's electrons in orbitals. For example:

What is a Bohr diagram?
The name of the rule stating that electrons will occupy the lowest energy orbital available: lower energy orbitals must be filled completely before starting to fill higher energy orbital.
What is the Aufbau rule or principle?