Where electrons are found
What are the electron cloud/outside of the nucleus?
Protons are represented by this number
What is atomic number?
This element has 20 protons and 19 neutrons. In hyphen notation
Calcium - 39
The amount of matter an object contains.
What is mass?
Isotopes are atoms that differ in this
What is an number of neutrons?
Nitrogen with 8 neutrons. In hyphen notation
Nitrogen - 15
What is mass over volume?
Found in the nucleus.
What are protons and neutrons
#Protons + #Neutrons
What is mass number?
Atomic number 13 and a mass of 28. In hyphen notation
aluminum - 28
Read the graduated cylinder.

What is 26.1mL?
This particle has virtually no mass.
What is an electron?
146 C and 136C are __________ of each other
isotopes
6 Protons and 8 neutrons. In hyphen notation
carbon - 14
The amount of space occupied by an object.
The atomic number is the number of this particle in the nucleus
What are protons?
This is the difference between positive and negative particles of an atom.
What is charge?
Sodium with a mass of 23 and 1 electron missing. In symbol notation with net charge
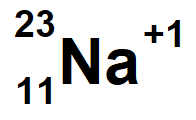
What is the name of the method used to determine volume of irregularly shaped things like rocks?
water displacement
What is the charge on each of the subatomic particles?
Protons - positive; electrons - negative;
neutrons - no charge
When atoms have a different number of electrons and protons.
What are Ions
Chlorine with 19 neutrons and an extra electron. In symbol notation with net charge
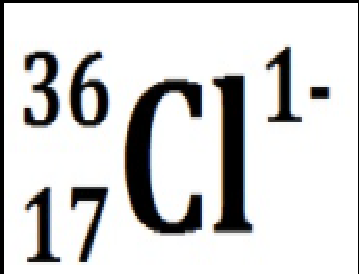
The collective name for protons, neutrons, and electrons.
What are subatomic particles?