These are the three subatomic particles that make up an atom
What are Proton, Neutron and Electron
This element has 44 protons.
What is Ruthenium?
Name the element and its number of valence electrons:
This scientist hypothesized this model for the atom:

Who is John Dalton?
These subatomic particles make up the nucleus.
What are protons and neutrons?
Titanium has this many protons, neutrons, and electrons.
What is 22 protons, 26 neutrons and 22 electrons.
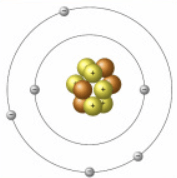
This is called the__________ model.
This is an atom that has a different number of protons and neutrons but retains its chemical properties.
What is an isotope?
206Hg has this many neutrons in one atom.
What is 126?
Name the element, protons, and electrons:
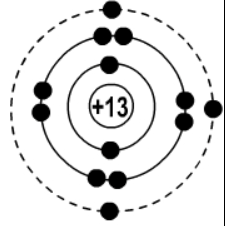
What is Aluminum 13 protons and 13 electrons?
This model shows electrons scattered through a cloud of positive charge.
What is the plumb pudding model?
These subatomic particles float around the nucleus due to electronegative repulsion.
What are electrons?
This atom has 54 protons, 78 neutrons, and 54 electrons.
What is 132Xe?
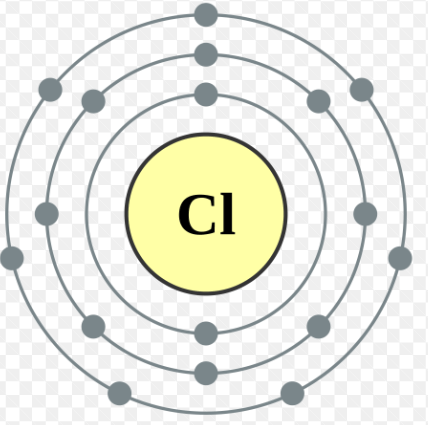
Draw the Bohr Model for the element Chlorine.
Rutherford conducted this experiment that lead to the discovery of the nucleus.
What is the gold foil experiment?
This is how you would determine the number of neutrons in an atom.
What is subtracting the atomic number from the atomic mass?
Bismuth has an atomic mass of 208.98, it has this many protons.
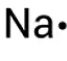
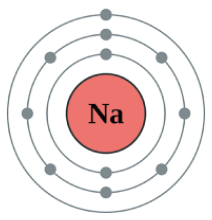
Put these models in order of discovery:
Quantum Model
Sphere Model
Nuclear Model
Planetary Model
Plumb Pudding Model
What is Sphere, Plumb Pudding, Nuclear, Planetary, and Quantum?