Because most particles fired at metal foil passed straight through, Rutherford concluded this about the inside of an atom?
Rutherford concluded that most of the atom was empty space with a dense center, which led to the discovery of the nucleus.
This subatomic particle determines the identity of an atom
Proton
What particle is gained or lost to make an ion?
electrons
Isotopes contain different numbers of what particle?
neutrons
If carbon has 2 peaks on the mass spectrum, at 12 and 13, which one will be larger?
12
There are this many electrons in an S-2 ion.
18
What is the charge of the nucleus?
positive
An atom is considered electrically neutral when?
Protons=electrons
How many subatomic particles are in the most common isotope of Fluorine?
9 p+ + 9 e- + 10 n0
28
What element is represented by this mass spectrum?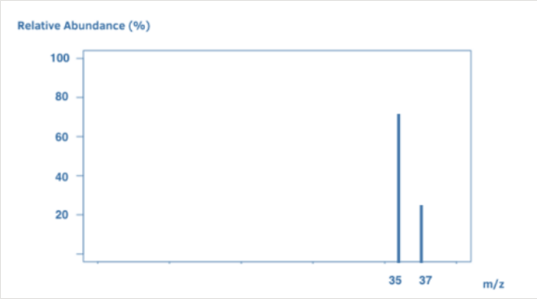
Chlorine
This is the nuclear symbol for Silver which contains 61 neutrons and has lost an electron.
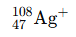
How many protons, neutrons and electrons are in the following:
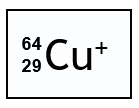
29 protons, 35 neutrons, and 28 electrons
What type of ion is K+ and how did it become charged?
Cation, lost electrons
An element X has two isotopes:
X-79 with a mass of 78.92 amu and an abundance of 50.7%
X-81 with a mass of 80.92 amu.
Calculate the average atomic mass of element X.
79.90 amu
A sample of neon is analyzed in a mass spectrometer. Three peaks appear at mass numbers 20, 21, and 22.
a) What do these peaks represent?
b) Which isotope is the most abundant in nature?
a) Peaks represent the isotopes 20Ne, 21Ne, and 22Ne
b)20Ne
An unknown element has two isotopes: 65 amu and 67 amu. The average atomic mass is 66.2 amu. What are the percent abundances?
65 amu = 40%
67 amu = 60%.
Element X is composed of two naturally occurring isotopes: 98.93% with a mass of 12 amu and 1.07% with a mass of 13.00335 amu. Calculate the average atomic mass and identify Element X.
Carbon 12.01 amu
How many protons, neutrons and electrons in Hg2+
80, 121,78
Z-101 with a mass of 100.95 amu and an abundance of 42.0%
Z-103 with a mass of 102.98 amu and an abundance of 17.0%
Z-104 with a mass of 103.99 amu and an abundance of 41.0%
Calculate the average atomic mass of element Z
102.54 amu
Magnesium has three isotopes:
24Mg (79% abundance),
25Mg (10%),
26Mg (11%).
A mass spectrum is obtained.
a)Describe what the spectrum would look like (relative peak heights).
b) Calculate the average atomic mass of magnesium.
a) Largest peak at 24, much smaller peaks at 25 and 26
b)24.32 amu