What element on the periodic table has 82 protons?
Lead
Identify the element that has 31 neutrons and a mass of 59.
Nickel
Who was the first scientist to propose a model of the atom?
John Dalton - Billiard Ball Model
How do you find the mass number of an atom if you only have protons, neutrons, and electrons?
Add the protons and neutrons
Ions are atoms that have a different number of __________ and __________
protons and electrons
What does the number 59 represent in the hyphen notation Nickel - 59
Mass Number
All atoms of the same element have the same _____________
number of protons
How many electrons can live on the first, second, third, and fourth rings of the bohr model?
2, 8, 8, 18
What is the mass of an atom with 10 protons and 9 neutrons, and 10 electrons?
Mass of 19
Isotopes of the same element have different _________ and _________
How many protons, neutrons, and electrons does and atom have if the atomic number is 33 and mass number of 75?
Protons 33
Neutrons 42
Electrons 33
What quantity can vary among neutral atoms of the same element?
mass number or neutrons
Which model of the atom shows electrons orbiting around the nucleus in fixed paths?
The Bohr Model
What is the subatomic particle that does not change within an atom?
Protons
How many electrons are in this ion?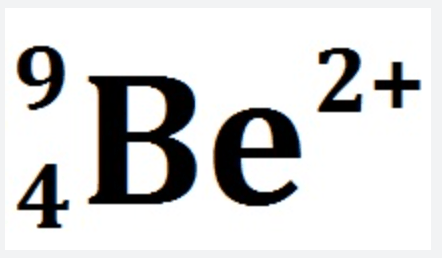
2 electrons
What does Atomos mean?
Indivisibile
What makes an atom electrically neutral?
Who discovered the electron?
J.J. Thompson
What is the number of neutrons in an atom of Carbon with a mass of 13?
7 Neutrons
If an ion of calcium has 18 electrons, what is the net charge on the ion?
+2
Which subatomic particles have the same mass?
Protons and Neutrons (1 amu each)
What is 1 atomic mass unit (amu) equal to?
1/12 the mass of a carbon 12 atom
What was the major discovery that lead to the development of the Quantum Mechanical Model?
Electrons have properties similar to waves and particles at the same time.
What is the number of protons in an element with a mass of 152 and 89 neutrons?
63 protons
If an ion of Tellurium has 54 electrons, what is the net charge on the ion?
-2