The number of protons in an atom of an element is its ___________ number.
atomic Number
__________ is the basic building block of all matter.
atoms
How many protons are in Boron?
5 protons
What is the Atomic Number of this atom?

7
Most of the periodic table is made up of
A. metals
B. nonmetals
C. metalloids
A. metals
What is the charge of a proton?
What is the charge of a neutron?
What is the charge of an electron?
Proton: Positive
Neutron: Neutral
Electron: Negative
What is the charge of a proton?
Positive
The number of neutrons in this element.

6
How many Electrons are in this atom?

2
The elements in Group 18 are?
A. metals
B. nonmetals
C. metalloids
Nonmetals
How many protons are in an atom of Cobalt?
27 protons
An element that has properties of both metals & nonmetals
metalloid
What is the atomic mass of Neon?
20
What is the Atomic Mass of this Atom?

14
What element is found at Group 2, Period 3?
Magnesium
What determines the identity of an element.
atomic number
A vertical column in the periodic table of the elements
group or family
How many electrons are found in Silver?
What is the Element Name of this (neutral) atom?

Oxygen
H2O is known as a
A. element
B. compound
compound
How many neutrons are in an atom of Phosphorus?
16 neutrons
What is the center of the atom called? And what is located in the center of the atom?
Nucleus. Protons and neutrons are located in the center of the atom
The number of neutrons in this element.
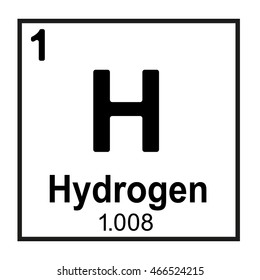
zero
What is the Atomic Mass of this atom?

23
What element is found in Group 17 and is located on period 5?
Iodine
A horizontal row in the periodic table of the elements
Periods
Vertical column that runs up and down.
What is a group?
How many neutrons does silver have?
61
How many Protons, Neutrons, and Electrons are in Nitrogen?
Protons = 7
Electrons = 7
Neutrons = 7
What is the maximum number of electrons that can be in the second energy level?

8
