The name for the center of an atom
What is the nucleus?
Atoms of the same element that have different masses
What are isotopes?
The charge on an atom with 18 protons, 20 neutrons, and 17 electrons
What is +1?
This element has 12 protons
What is magnesium (Mg)?
The subatomic particles in the nucleus
What are protons and neutrons?
Isotopes have different numbers of these particles
What are neutrons?
The charge on an atom with 15 protons, 17 neutrons, and 18 electrons
What is -3?
The name for a charged atom
What is an ion?
The subatomic particles that have charge
What are protons and electrons?
The number of neutrons in carbon-12
What is 6?
The number of neutrons in an atom of Na-23
What is 12?
This neutral atom has 36 electrons
What is krypton (Kr)?
The location of an atom where electrons are most likely to be found
What are the electron cloud, shells, or orbitals?
The number of neutrons in carbon-14
What is 8?
The number of neutrons in an atom of Si-30
What is 16?
The name for electrons jumping to a higher energy level
What is an excited state?
The subatomic particles that have mass
What are protons and neutrons?
The most common (naturally abundant) isotope mass for oxygen
What is 16?
Which two particles are isotopes?
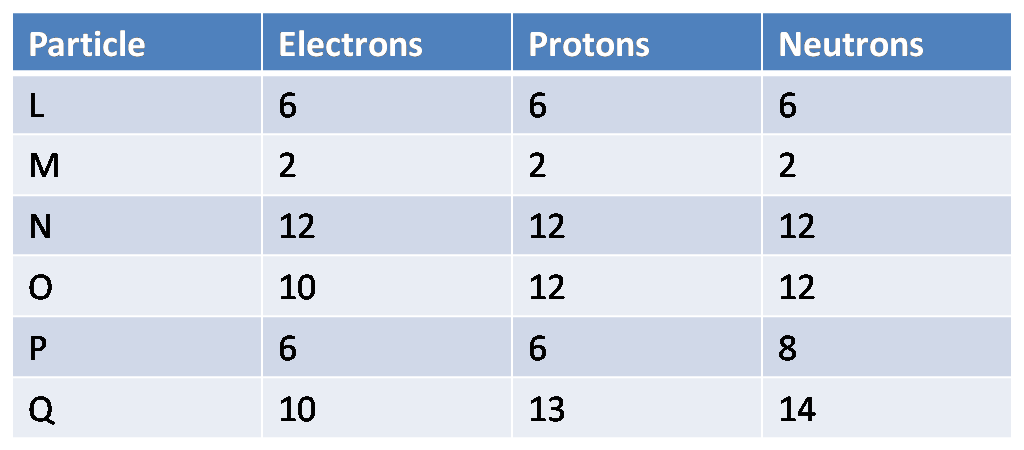
What are L and P?
Name a gas that is present in the mixture:
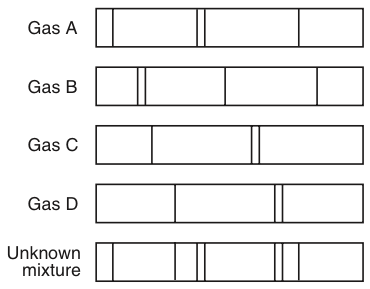
What are gas A and gas D?