Proton: positive (+1)
Neutron: neutral
Electron: negative (-1)
An isotope is defined as what?
An atom of an element with the same number of protons, but different number of neutrons
A hydrogen atom has 1 proton and 2 neutrons. What is its name?
Hydrogen-3
Alpha: Helium nucleus
Beta: Electron
Gamma: Energy
Below is an example of which radioactive decay?
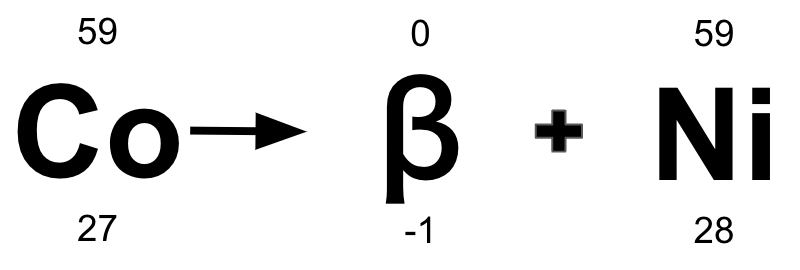
Beta Decay
What is the location of each subatomic particle within the atom?
Outside Nucleus: Electrons
Unstable isotopes are also known as what?
Radioactive
A sulfur atom has 16 protons and 18 neutrons. What is its name?
Sulfur-34
Which radioactive decay results in an increase in atomic number for the daughter product?
Beta decay
Below is an example of which radioactive decay?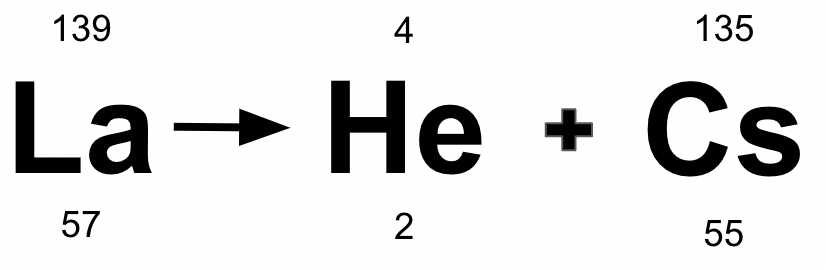
Alpha Decay
Which particles determine the atomic number and the mass number of an atom?
Atomic: protons
Mass: protons and neutrons
The name of an isotope includes the name of the element followed by what?
The atom's mass number
32 neutrons
Which form of decay results in a net loss of protons?
Alpha decay
Which type of decay is occurring, and what is the missing element/atomic mass/atomic number?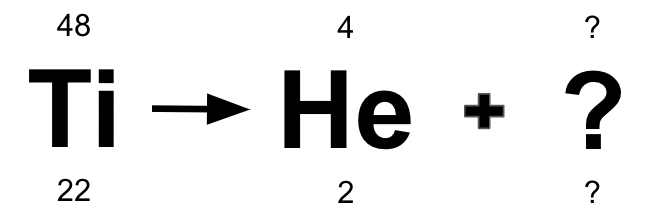
Alpha Decay
Calcium/44/20
Which particle determines an atom's stability and which particle determines an atom's neutrality?
Stability: neutrons
Neutrality: electrons
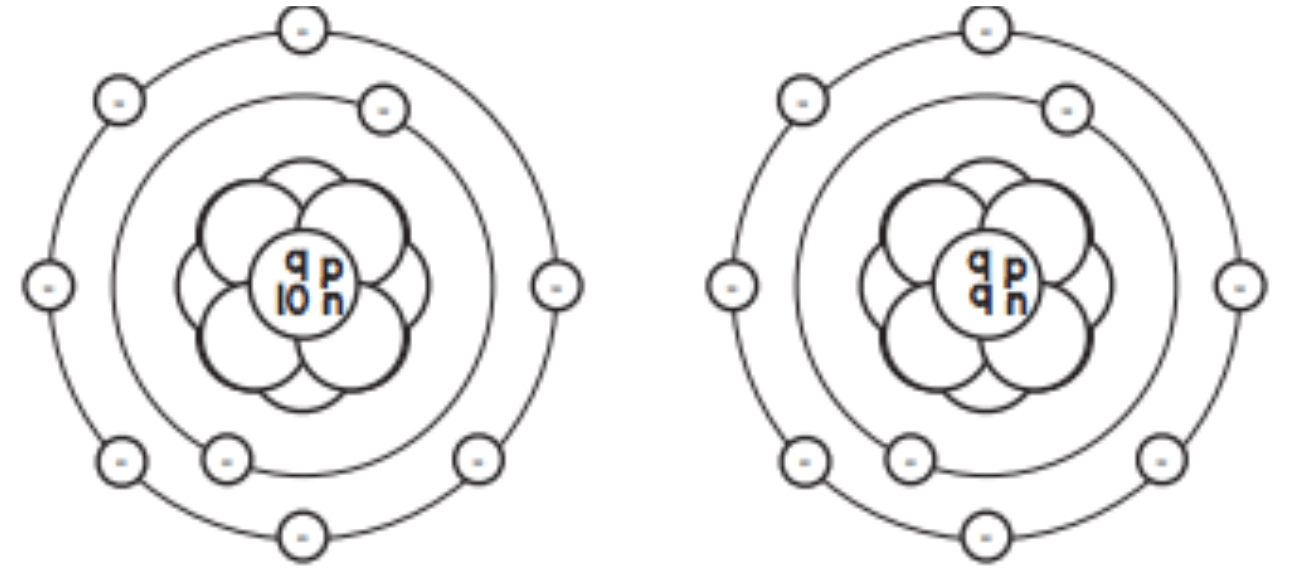 Are the above two atoms isotopes? Why or why not?
Are the above two atoms isotopes? Why or why not?
Yes they are isotopes: they have the same number of protons, but different numbers of neutrons
An atom of Gold-197 has 118 neutrons. What is its atomic number?
79
Which form of decay results in a net loss of neutrons?
Both Alpha and Beta
Which type of decay is occurring, and what is the missing element/atomic mass/atomic number?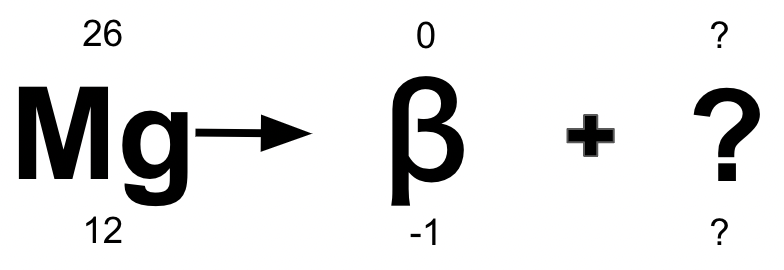
Beta Decay
Aluminum/26/13
What is the difference between an atom's mass number and an element's atomic mass?
Mass number: total number of protons and neutrons in one atom of an element
Atomic mass: the average of every mass number of every atom of a given element
Atoms 1 and 3
An atom with 30 neutrons has an atomic number of 26. What is the name of this isotope?
Iron-56
What is the difference between Alpha and Beta decay in what happens to the neutrons that they lose?
Alpha: lost in the alpha particle that is shed
Beta: transformed into a proton and electron
Based upon this graph, which element has undergone radioactive decay, which form of decay, and how do you know?
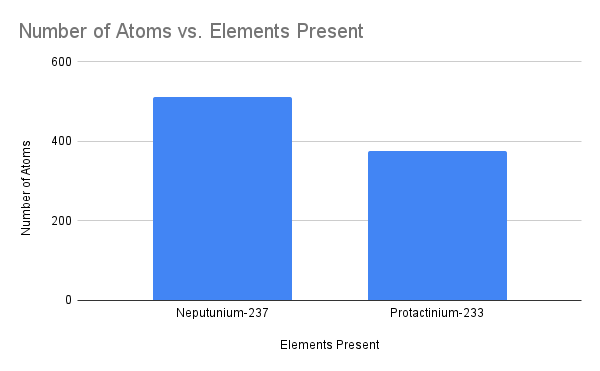
Neptunium-237 has undergone alpha decay; Protactinium-233 has a mass number 4 less and an atomic number 2 less than, Np-237. This indicates alpha decay