What is the smallest unit of an element that retains all of the properties of that element?
Atom
an atom with an electrical charge is called what?
ion
Isotopes of the same element have different what?
masses or different number of neutrons
ancient Greeks believed that all matter was made up of what 4 elements?
fire, water, earth and air
what does the Greek word "atoms" mean?
indivisible
the name of the negative charged part of an atom
electron
what is a negatively charged atom called?
an anion
What does the number 235 represent in Uranium-235?
the mass number
J.J. Thomson
Plum pudding model
What is the name for Dalton's atomic model?
The billiard ball model
These two atomic particles account for an atom's mass
protons and neutrons
what is the name of a positively charged atom?
a Cation (it's paw-sitive)
How many neutrons does Uranium-235 have?
143
they have a nucleus surrounded by electrons and are made up of mostly empty space
what is the capital of Texas?
Austin
 what is the name of the blue region in this diagram?
what is the name of the blue region in this diagram?
electron cloud
If an atom is neutral, how would you show that on a nuclear symbol?
Leave it blank
what type of notation is used when you give the elements name followed by its mass?
hyphen notation
Antoine Lavoisier is credited with creating what law and what does that law state?
The law of the conservation of mass - in a chemical reaction the mass of the reactants equals the mass of the products (mass is neither created or destroyed)
What element on the periodic table has 40 protons?
Zirconium
Name the particles and their charges of an atomic nucleus
proton - positive
neutron - neutral
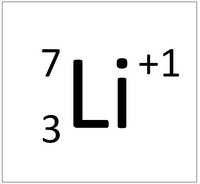
create a nuclear symbol for a lithium ion that lost one(1) electron
true or false:
Isotopes of the same element will react differently in chemical reactions
false
what principle states that it is impossible to simultaneously know an electron's position and velocity?
the Heisenberg Uncertainty Principle
1/12 of the mass of carbon-12 is equal to what?
1 amu (atomic mass unit)