What is the largest planet in our solar system?
Jupiter
What family does Sodium belong to on the periodic table?
group 1, alkali metals
Name one property of the transition metals.
they are good conductors of heat and electricity.
they can be hammered or bent into shape easily
they have high melting points (but mercury is a liquid at room temperature)
they are usually hard and tough.
they have high densities.
Like to lose 1, 2, or 3 electrons to have a positive charge (more on this later)
What are the three main particles found in an atom?
protons, neutrons, and electrons?
what is another name for electron orbitals?
electron shells, energy levels
What is the duet rule? AND What is the octet rule?
DUET RULE = ONLY TWO ELECTRONS IN VALENCE SHELL
OCTET RULE = 8 ELECTRONS IN VALENCE SHELL
How many protons does Xenon have?
54
Who wrote the play "Romeo and Juliet"?
Shakespeare
What does it mean for a metal to be malleable?

What is the charge of a proton?
positive
How many electrons can the first 3 electron shells hold?
1st - 2
2nd - 8
3rd - 8
How can we easily figure out how many valence electrons an element has?
looking at the GROUP NUMBER of that element
How many electrons are in a neutral atom of Iron?
26
Which family does Calcium belong to in the periodic table?
group 2, alkaline earth metals
What is the capital city of France?
Paris
Where are protons and neutrons located in an atom?
nucleus
What is the outermost electron orbital called?
valence shell/orbital
Which elements want to GAIN electrons to fill their valence shells?
groups 5, 6, 7
What is the number of neutrons in Phosphorus?
16
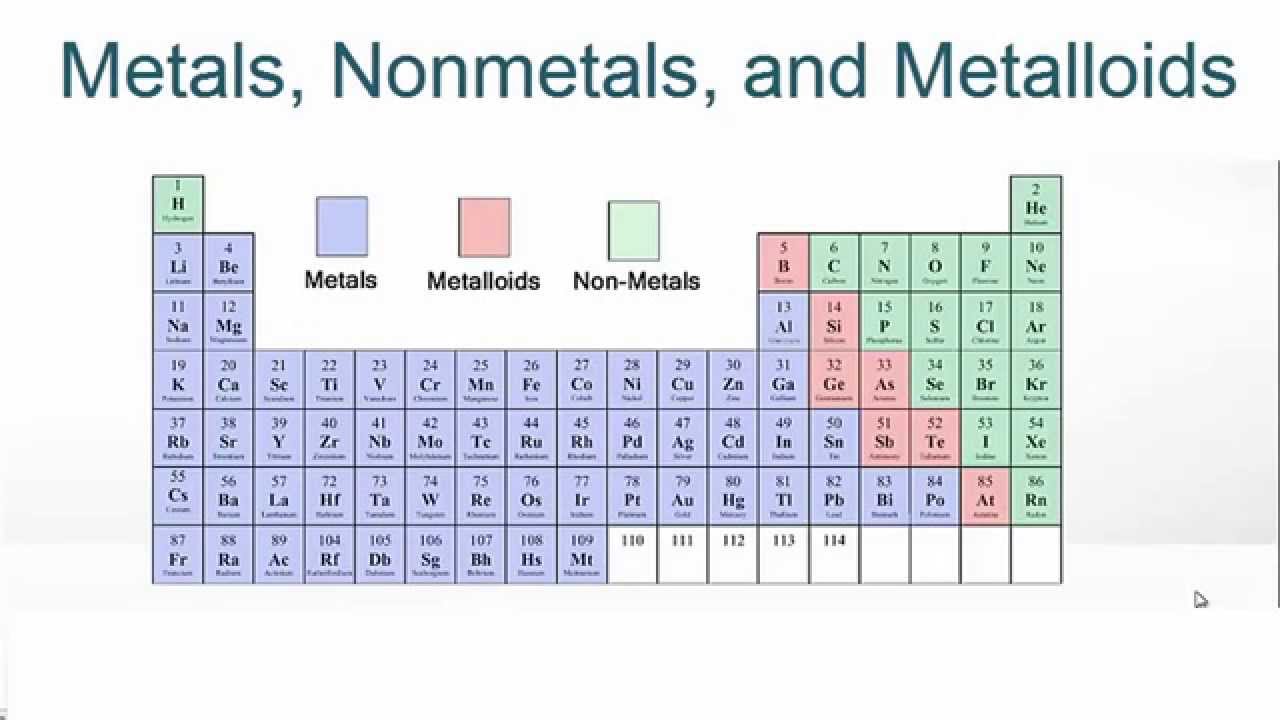
Where are metals located on the periodic table?
to the left of the staircase
Give three examples of metals from the periodic table
Which ocean is the largest?
Pacific
What determines the behavior of an atom AND what does "behavior" mean?
The valence electrons determine the behavior of the atom.
Behavior = how reactive the atoms are. This means how likely they are to want to bond with other atoms.
Which elements want to LOSE electrons to have a full valence shell?
groups 1, 2, 3
Calculate the mass number of Chlorine, given its protons, electrons, and neutrons.
35.5 amu
Where are metalloids on the periodic table?
touching the staircase
give three properties of a nonmetal
Non-metals can be gases, liquids or solids.
Non-metals are dull in colour, not shiny like metals.
You can't hammer or shape a non-metal; it will just shatter if you hit it.
What determines the identity of an element?
the number of protons
Do most atoms have a full valence shell AND How do atoms fill their valence shells?
Most atoms DO NOT have a full valence shell. But they really WANT to have a full valence shell.
In order to have a full valence shell, atoms will bond with other atoms to help each other have full valence shells.
How can you tell the number of electron shells that an element has?
look at the row or period number.