What is the mass and charge of a proton?
1 amu / +
The number of protons and neutrons in an atom added together is the __________.
mass number (or atomic mass)
Where are neutrons located?
Inside the nucleus
What is the mass of an electron?
Pretty much weighs nothing (0.0005 amu) or (9.10938356 × 10-28 grams)
What is the formula used to find the average atomic mass of an element?
Av Mass = (% X mass) + (% X mass)...
How many protons, neutrons, and electrons does Magnesium-25 have?
P = 12
N = 13
E = 12
Where are electrons located?
In energy levels around the nucleus (would also accept orbitals)
What is the overall charge of an atom?
Neutral: the number of electrons equals the number of protons
What are three pieces of information you can gain from knowing the atomic number of an atom?
The number of protons, the number of electrons, and the identity of an element.
How many protons are there in O-87?
8 protons (oxygen ALWAYS has 8 protons. If it didn't it wouldn't be oxygen)
What is the machine called that can separate isotopes by their weight?
mass spectrometer
I have three isotopes of carbon. What do they have in common? How do they differ?
They have the same number of protons, but different number of neutrons (or a different masses)
How many neutrons are there in Na-30?
19 neutrons (30 is the mass number - atomic number (11) = 19)
How many neutrons does the most abundant isotope of Aluminum have? (round accordingly)
14 neutrons (Weighs 27 amu - 13 protons = 14 neutrons)
Electrons orbit the nucleus in the electron cloud. Where (in the cloud) will there be the greatest chance of locating an electron?
Closest to the nucleus.
When atoms enter a mass spectrometer, they are stripped of an electron. What does this do to the overall charge of the atom?
It makes it have a +1 charge (because now the # of p+ and e- are not equal).
If the mass number of an atom is 23, and the atomic number is 11, how many neutrons does it have?
12
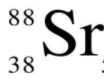 How many protons, neutrons, and electrons does this isotope of Strontium have?
How many protons, neutrons, and electrons does this isotope of Strontium have?
protons = 38
neutrons = 50
electrons = 38
I have not taught you this *technically*, BUT can you figure it out.... How many protons, neutrons, and electrons does this isotope of strontium have?
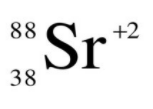
protons = 38
neutrons = 50
electrons = 36
(the 2+ charge indicates that there are two more protons than electrons)