Which subatomic particles contribute to atomic mass?
protons & neutrons
Which particle determines the identity of an element?
Protons
What is the term for when a nucleus undergoes a change?
Transmutation
Who said you cannot know both the momentum and the location of an electron at the exact same moment?
Heisenberg
Who was the first person to predict properties of unknown elements based on their positions on the periodic table?
Mendeleev
Which type of orbital can hold a maximum of 6 electrons?
p
How many protons, neutrons and electrons are in a neutral atom of phosphorus?
15 protons
16 neutrons
15 electrons
What is the charge of an element that has gained two electrons?
-2
Write the hypen and nuclear symbol notation for an element with 90 protons and 140 neutrons.
Thorium - 230. 23090Th
Bohr
Which element on stair-step line is NOT a metalloid?
Aluminum
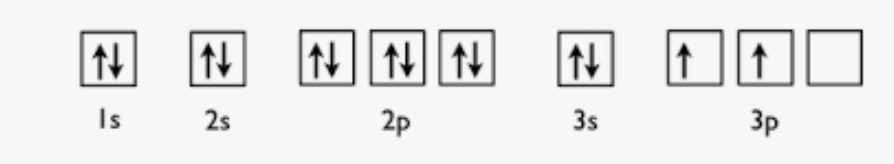
Draw and label the orbital notation for silicon
What are protons and neutrons made of?
Quarks
Why do most atomic mass values on the periodic table contain decimals?
They are the average of all the naturally occurring isotopes of that element.
6.25%
What idea says that two electrons in the same element cannot have the exact same set of four quantum numbers?
Pauli Exclusion Principle
Which group on the periodic table is highly reactive and is likely to lose one electron when they bond?
Alkali metals
Write the electron configuration for Manganese.
1s2 2s2 2p6 3s2 3p6 4s2 3d5
What is the largest occurring natural element?
Uranium
Sodium - 22; +1 charge
What is the energy given off during radioactive decay?
Gamma ray
John Newlands noticed a repeating pattern when elements were arranged by atomic number. What is the name of this patter?
Law of Octaves
Actinides
[Xe] 4f14 5d6 6s2
How many atoms are in 64grams of an element with 16 protons? Show your dimensional analysis
(64 grams S)/ 1 xx (1 mol)/ (32.0grams S) xx (6.02 x 10^23 atoms)/ (1 mol) = 1.2x10^24 atoms
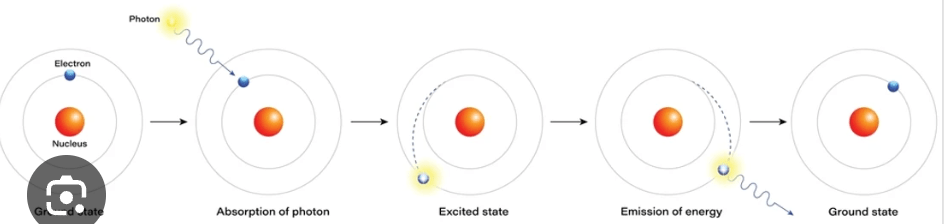
Draw and label a diagram that shows what happens to electrons in an atoms when they are exposed to high powered heat or electricity.
Complete the following equation and identify the type of decay particle.
146C --> ______ + 0-1e
147N Beta particle
Describe the trend in atomic radius as you go down a group and across a column AND predict the atomic radius of Rubidium.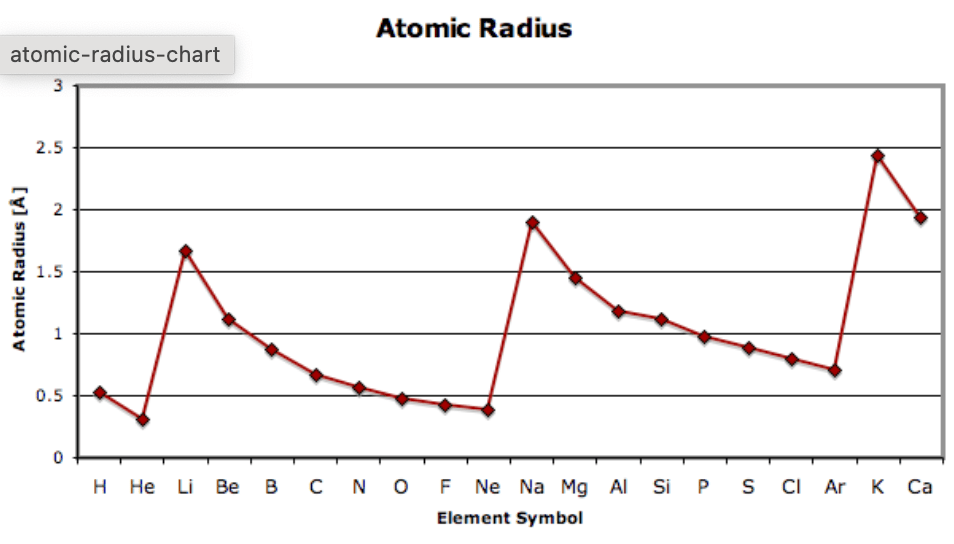
Down a group - increases
Across a period - decreases
Rubidum - atomic radius ~3.
Which group has the a different principle quantum numbers but the same angular, magnetic and spin numbers of X, 1, 0 -1/2
Hallogens
Palladium, Pd