Who discovered the plum pudding model, suggesting atoms have negatively charged electrons in a positive "pudding"
J.J. Thomson
The charge of an electron
negative
What does the number 56 equal in the hyphen notation Iron-56?
What is Atomic Mass
What two forms can identify an isotope
Hyphen form and nuclear form
What is an electron?
A subatomic particle located in the electron cloud surrounding the nucleus that has a negative charge
Who came up with the idea of atomos: all matter is made up of small indivisible particles
Democritus
The location of protons in an atom.
What is the nucleus?
Where is most of the atom's mass located?
What is in the nucleus?
32S has how many neutrons?
16
What is an atom?
The smallest particle of an element which retains the properties of that element
(building blocks of matter)
Which scientist created a model of the atom where there is a nucleus in the center surrounded by rings that the electrons orbit on? 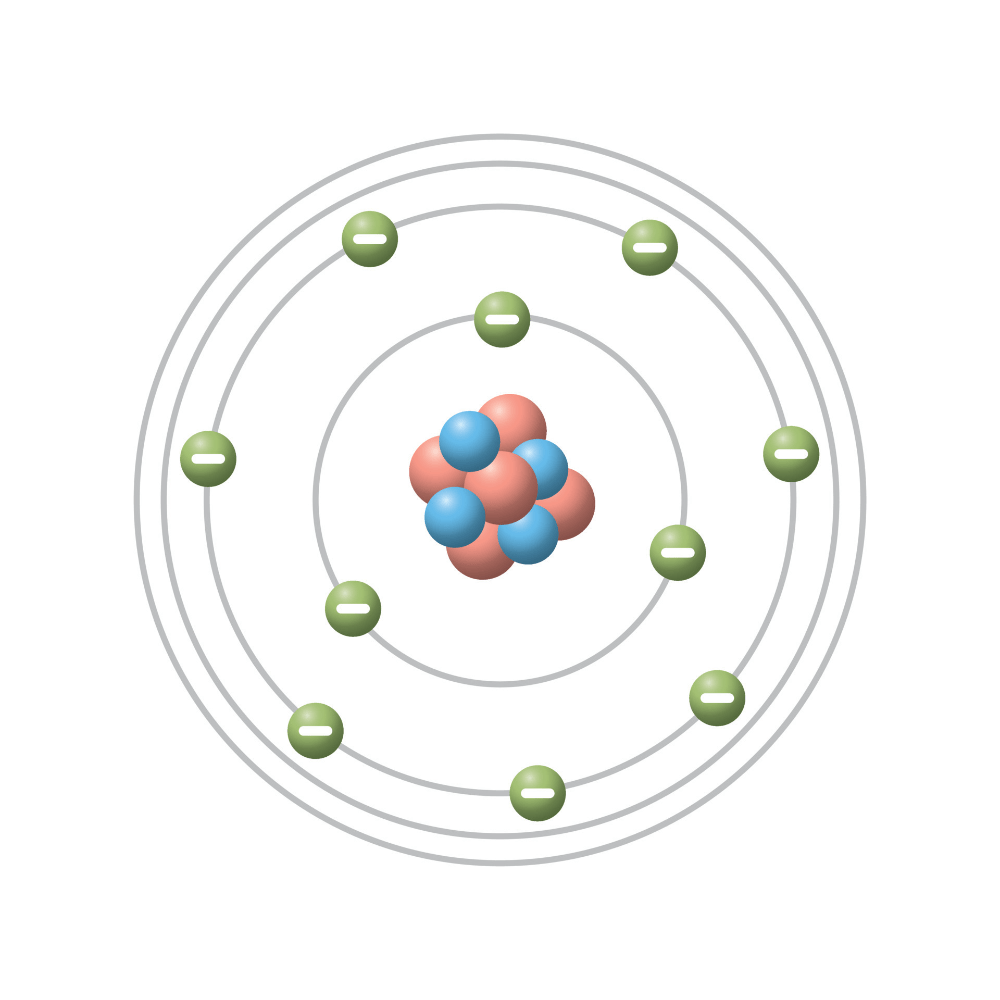
Who is Niels Bohr or Bohr Model?
The way to calculate the mass number.
What is atomic number + neutrons?
What is an isotope?
atoms of the same element with different numbers of neutrons
The mass of an atom with 10 protons and 9 neutrons, and 10 electrons
What is 19?
Where are electrons located?
the electron cloud
Who said that each element is made of atoms that are all alike?
John Dalton
Describe the different subatomic particles of an atom
protons in the nucleus (positively charged)
neutrons in the nucleus (no charge)
electrons in the electron cloud (negatively charged)
Particles that make up protons and neutrons are known as
Quarks
What is the number of protons in an element with a mass of 152 and 89 neutrons
63
What is the nucleus?
located in the center of the atom (consists of protons and neutrons)
What model states electrons form a negatively charged cloud around the nucleus?
Present Modern Model
The mass of the neutron or proton.
What is 1 amu?
The weighted average of all the isotopes of that element
What is the average atomic mass?
What is the mass of an isotope of Hydrogen with 2 neutrons
mass is 3
What are valence electrons?
electrons in the element's outermost energy level
Who proposed that electrons orbit randomly around a small, positively charged nucleus
Rutherford
The mass of electrons
almost no mass
1/2000 amu
Isotopes of the same element have different _______________
What is masses or neutrons?
How many neutrons are in Carbon-13
7 neutrons
What is an element?
matter composed of one type of atom