Three subatomic particles in the and their charges.
What are protons (+ charge), electrons (- charge) and neutrons (0 charge)?
The number of protons in an atom.
What is atomic number?
The definition of an isotope.
What are atoms of the same element with different numbers of neutrons?
This number on the periodic table shows the number of protons in an element's atoms.
What is atomic number?
What is mass # = protons + neutrons?
So what is mass # - protons = neutrons?
Protons, neutrons and electron locations in the atom.
What is the nucleus for protons and neutrons and what is the electron cloud (in orbitals) for electrons?
Protons + Neutrons - a whole number
What is mass number?
To create an isotope, the number of these subatomic particles changes.
What are neutrons?
This number on the periodic table measures the mass of an atom, which mostly consists of protons and neutrons. It is also a weighted average of the masses of an element's isotopes.
What is atomic mass?
An atom has an atomic number of 15 and a mass number of 31. This atom has ________ neutrons.
mass # - protons = neutrons
31 - 15 = 16 neutrons
The number of these particles determines the identity of an element (its name)
What are protons?
What is 50?
This is how isotopes of the same element are similar and how they are different.
What are the similar - number of protons and what are different - neutrons and mass numbers.
75% of chlorine (Cl) atoms are the isotope Cl-35 and 25% are Cl-37. Of these two isotopes, this one impacts the atomic mass more.
What is Cl-35?
The nuclear symbol for this element is below. It has _____ protons, ______ neutrons and a mass number of ____________.

What is helium with 2 protons, 2 neutrons and a mass number of 4.
The relative masses in amu of protons, neutrons and electrons.
What is approximately 1 amu each for protons and neutrons and what is approximately 0 amu for electrons? (The point is, protons and neutrons make up most of the atom's mass, while electrons are so small their mass is very close to 0 amu)
These elements from lowest to highest atomic number.
Rb, Sn, Br, N
What is: N<Br<Rb<Sn
This atom has 12 protons and 10 electrons. It is (an ion or neutral) with a charge of ____________.
What is an ion with a charge of +2. (Originally, the atom had 12 protons and 12 electrons and was neutral. Then it lost two electrons, making it more positively charged than negatively charged. Practice creating positive and negative ions on the Build the Atom simulation)
What is the weighted average of the isotopes of that element?
The atom below is (an ion or a neutral atom) and has _____ protons and _____ electrons.
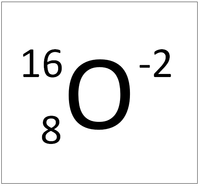
What is an ion with 8 protons and 10 electrons. (Remember, it is the electrons that change to form an ion)
Loss or gain of one or more of these particles leads to formation of an ion.
What are electrons?
Difference between atomic mass on the periodic table and the atomic mass of a single isotope.
What is the weighted average the mass of isotopes of an element versus the mass of a single isotope.
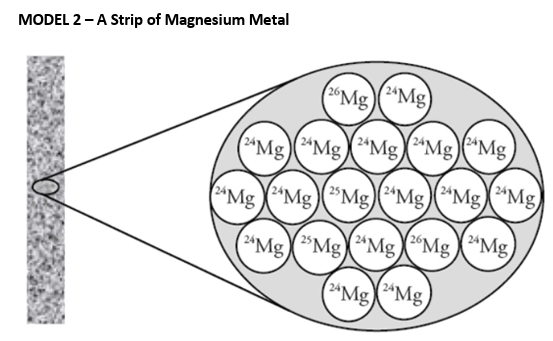
Out of these three atoms - atom A (6p, 6n), atom B (6p, 8n) and atom C (7p, 7n), these two atoms are isotopes.
What are atom A and atom B?
Using atomic mass data, this is how you could approximate the mass of the most common isotope of an element.
What is the most abundant isotope that has an atomic mass closest to the value on the periodic table?
This chromium atom has this many electrons. Use your periodic table.
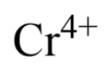
On the periodic table, a neutral chromium atom has 24 protons and 24 electrons. This atom has a symbol of 4+, which means it has lost 4 electrons. So it has 24 - 4 = 20 electrons.