Protons + Neutrons=?
What is the mass number?
Half-life of Caron-14 based on the graph:

What is an electrically-neutral atom?
Charge and location of an electron, proton, and neutron.
What is -1 and outside the nucleus?
What is +1 and inside the nucleus?
What is no change and inside the nucleus?
Atoms of the same element with different numbers of neutrons.
What is an isotope?
The time it takes for half of the original amount of the sample to decay.
What is half-life?
Predict this element based on the image:

What is Neon?
Protons in the nucleus for Nitrogen and give the other name for the number of protons.
What is 7 and the atomic number?
Find the identify of this electrically neural atom based on the data:
mass number=91
electrons=40
Who is zirconium?
Identify the number of protons, electron, and neutrons for a Calcium-41 atom.
What are 20p,20e and 21n?
If unstable, this part of the atom undergoes radioactive decay.
What is the nucleus?
The term for the? and describe their charges.

Who are quarks?
quarks up and quarks down
Describe what is common between these isotopes:
Scandium-45
Titanium-46
What is the same number of neutrons?
Answers can vary
Particle that establishes the identify of an element.
What is the proton?
Three things about the particles in Cl-35 and Cl-37.
What is the same number of protons and electrons, but different neutrons for these isotopes of Cl?
Answers can vary
The average atomic mass of boron as listed on the periodic table is 10.81 amu. Predict which isotope is more abundant and why.

What is boron-11 because its mass number is closer to the avg atomic mass on the periodic table?
Complete the transmutation equation shown below:

What is 231 Th ?
90
If an element X has atomic number 29 and isotopes with mass numbers 63 and 65, state how many neutrons are in each isotope.
What is 34 and 36 neutrons?
The number of protons and neutrons for a Chromium-52 atom.
What is 24p and 28n?
These two things are needed in order to calculate a weighted average.
What is the % abundance and mass number(exact mass)?
Describe radioactivity in terms of subatomic particles.
When an atom has too many protons or neutrons?
Answers can vary
Describe this image:

Questions can vary
Describe the weighted averages for an element that appear on the periodic table.
What is an avg that takes into account the % abundance of each isotope for an element?
Can vary
Name the element and charge depicted in this image:
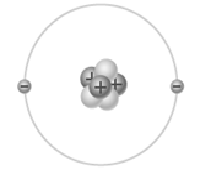
What is Lithium (Li) and +1?
Describes the data in the image:

Answers can vary

What are gamma rays or option D?