The positively charged particle
A proton
Double points
A (n) _____ is a very small particle that is the basic unit of matter.
Atom
How many atoms do you need to form a molecule?
2 or more
Double points
How many electrons does an atom of Helium have?
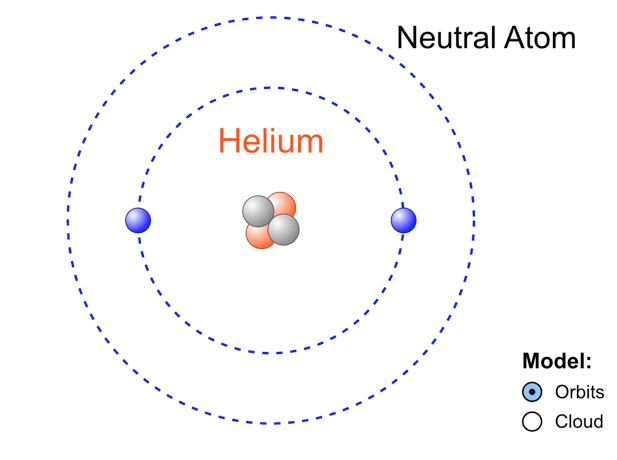
2
 What is the Atomic Number of Silicon?
What is the Atomic Number of Silicon?
14
The number of protons in an atom of an element is the same as its number of ______
electrons
Double points
This particle moves around the outside of nucleus
electrons
In the molecule H2o, what do the letters represent? What about the number?
The letters are the type of atom (hydrogen and oxygen) and the number tells us that there are 2 hydrogen atoms in the molecule.
Double points
What is the difference between mass and volume?
1) Mass is the weight
2) volume is the amount of space something takes. up
What is the number of electrons in Silicon?

14
What is in the nucleus of an atom?
protons + neutrons
Neutron have _______ charge
zero / neutral
Double points
This is a connection between atoms that holds molecules together
bonds
 How many electrons does a Beryllium atom have?
How many electrons does a Beryllium atom have?
4 electrons
Double points
What is the Symbol of Silicon?

Si
Double points
What is most of an atom made of?
Blank space
These are things that are made of two or more atoms
molecules
Double points
What is special about water compounds?
The hydrogen and the oxygen atoms do not share electrons equally, so water is a polar molecule.
How can two substances be made of the same element but look and feel different?
The arrangement of their molecules are different
How many neutrons are in Silicon?

14 or 14.086
What does the Atomic Mass - Atomic Number equal?
the # of neutrons
A solid material whose atoms, molecules, or ions are arranged in an ordered pattern.
Crystal
Why is salt shaped like crystals?
The atoms are arranged in a specific pattern.
What is matter?
Anything that has mass and volume
What is the "Zn"? or any letter on the periodic table
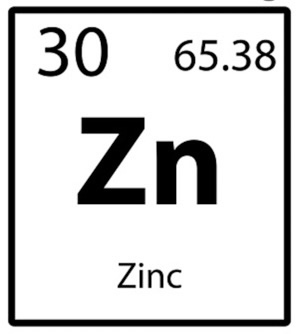
Chemical symbol
What part of an atom bonds to another atom?
Electrons
Double points
What is a subscript?
A number written to the right of a chemical symbol to show how many of that specific type of atom are present.
What is an extended structure?
A substance with molecules that are repeated in the same pattern over and over again.
What can substances be made of?
individual atoms or groups of molecules.
What is the Atomic Number, Atomic Mass and Number of Protons, Electrons, and Neutrons in Cobalt?

Atomic Number: 27
Atomic Mass:58.933 or 59
# of protons: 27
# of electrons: 27
# of neutrons: 31.933 or 32
Double points