Number of protons, electrons, and neutrons in a vanilla sulfur atom.
16 protons, electrons, and neutrons
The difference between atomic mass and mass number.
Mass number is a whole number that is the sum of protons and neutrons.
Atomic mass is a decimal number that is calculated using abundances and mass numbers of isotopes.
What are the rows and columns on the periodic table called?
Rows are periods.
Columns are groups.
This family of elements always has a full valence shell.
The noble gases
Which element has a smaller atomic mass, Ca or O?
O
An atom with 15 protons, 16 neutrons, and 18 electrons is this type of atom.
An ion and an isotope
What do you have to do to percent abundance when using it to calculate atomic mass?
Divide by 100 to get it to a decimal.
Move the decimal left 2 times.
The halogens are considered what on the periodic table.
A family.
What is the same for the electron configuration of all elements in the 3rd period?
They have 3 energy levels. Their valence shell is 3.
Between Cs and Li, which element has the lower ionization energy?
Cs
An element that has 4 protons, 4 neutrons, and 2 electrons has a charge of what? What type of atom is this?
+2 charge. It is an ion
Boron has 2 isotopes. Boron-11 with a percent abundance of 80.1% and Boron-10 with a percent abundance of 19.9%. What is the name of the most common isotope without calculating the atomic mass?
Boron-11
The third period of the metalloids is this element.
Silicon
What is the valence shell and number of valence electrons for Aluminum?
Valence shell is 3, valence electrons is 3
Put these elements in order from lowest to highest electronegativity: F, P, Cl
P, Cl, F
26 protons, 23 electrons, 26 neutrons
If tin's atomic mass is 118.7. What is the mass number of its most common isotope?
119
This family is found outside of the normal periodic table but fits in row 6 and row 7 of the table.
Rare earth metals.
Scandium
Why do noble gases have high ionization energy but zero electronegativity?
They already have a full valence shell, so they do not need to gain more electrons. This also means it takes a lot of energy to remove their electrons
Write the atomic notation for an atom with 34 protons, 42 neutrons, and 36 electrons.
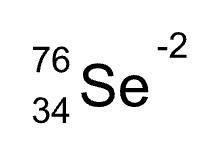
Calculate the atomic mass of copper using the information below
Copper-63 69.2%
Copper-65 30.8%
63.62
Why does the first period only have 2 elements, while the 2nd period has 8?
Based on the number of electrons that fit on each energy level.
What is the electron configuration of Krypton?
2e, 8e, 8e, 18e
Put these elements in order from smallest to largest atomic radius: Re, Zn, Hg, Mn