the 3 subatomic particles
proton, neutron, and electron
Two or more atoms bonded together by chemical bonds is called a
Molecule
These are vertical columns on a periodic table
groups
How many protons are in the following element?

36
What is an isotope?
Elements of atoms with the same number of protons but different neutrons
the location of protons and neutrons
the nucleus
This number tells you the number of protons
the atomic number
These are horizontal rows on a periodic table
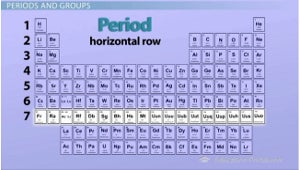
periods

Two isotopes of Magnesium are Mg-24 and Mg-26. How are these isotopes different from each other?
The number of neutrons and atomic mass
Which sub atomic particle has the lightest mass?
electrons
The atomic number is equal to what?
protons
An element is in period 3, group 18 and in the noble gas family. Would it be classified as a metal, nonmetal or metalloid?
nonmetal
The atomic mass or mass number tells us how many __________ an element has in its nucleus.
protons and nuetrons
WHats the main difference between hydrogen and helium?
a proton
What charge does a nuetron have?
nuetral
What element is in period 3, group 14
3
![]()
Name 1 element that acts similar to Ar
helium, neon, krypton, xenon or radon
How many neutrons does the following element have?

16
How is the periodic table arranged by?
Atomic number mainly
How many electrons does the element potassium have?
19
How many neutrons are in berylium (Be)
5
This is the number of neutrons in the element Cl (chlorine).
18
Name one gas that has more than 10 protons
Iodine, Carbon, Sulfur, Silicon, Magnesium, Aluminum
Which of the following elements have similar properties to Magnesium?
Calcium, Lithium, Sodium
Calcium (Since elements in the same group/family have similar chemical properties. )
What is the amount of nuetrons in isoptope Iodine 127?
74