What are the three subatomic particles of an atom?
What are protons, neutrons, and electrons?
The number of neutrons in this element.

What is 6?
A subatomic particle that has no charge.
What is a neutron?
A salad is an example of this type of mixture.
heterogeneous
I’m a gas that’s light as air, My symbol is H, and I’m everywhere!
hydrogen
Electrons are found here.
What is the electron shells?
The atomic weight of this element.
What is 55.845
A negatively charged subatomic particle
What is an electron?
True or false? Mixtures are chemically combined.
false
I am essential for life and make up about 65% of the human body. What am I?
oxygen
In an atom, which has the least mass?
What is D: electron
The number of neutrons in this element.
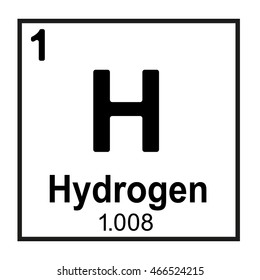
What is zero?
The mass number of an atom with 3 protons, 4 neutrons and 3 electrons is this.
What is7?
A pure substance is made up of two or more substances
false
I’m essential to living things and the main player in organic molecules. Who am I?
c
Protons and neutrons are found here.
What is the nucleus.
What determines the identity of an element.
What is the number of protons or atomic number?
The area around the nucleus of an atom where electrons are most likely to be found?
What is an Electron cloud
Two different elements chemically combined.
compound
Why did the element go to the party?
Because it was a bonding experience!
This number tells us what the element is
Atomic Number or Number of Protons
Define an element
pure subtance that cannot be changed
The overall charge of a whole atom is this.
What is zero.
SOLUTE
SOLVENT
the atom is mostly
empty space