__________ are the basic building block of all matter.
What are atoms?
The number of protons in an atom of an element is its ___________ number.
What is atomic?
The addition of this subatomic particle makes an element an isotope
Neutrons
A positively charged subatomic particle.
What is a proton?
All of the periods (rows) have this in common
What are number of energy levels?
This subatomic particle is neutral
Neutrons
What group of elements is nonreactive

Group 18 noble gases
Name a characteristic of Group 18.

Atom has a full valence electron shell and is not reactive
When looking at the periodic table, the vertical columns are
What is a group?
The sum of protons and neutrons in the nucleus.
What is : atomic mass
Electrons are found here.
What are energy levels
Rows of elements are called this.
What are Periods?
The atomic weight of this element.
What is 10.81
A negatively charged subatomic particle
What is an electron?
The addition of this subatomic particle makes an atom a ion
Electrons
In an atom, which has the least mass?
A: nucleus
B: proton
C: neutron
D: electron
What is D: electron
Name the 2 types of ions
cations and anions
What element has atomic number 6

Carbon
Elements found in the same group (column) have the same..
The answer, my good sir, is the same number of VALENCE ELECTRON NUMBER?
A fluorine atom has 9 protons, 10 neutrons, and 9 electrons. What is the isotope name
Protons and neutrons are found here.
What is the nucleus.
The number that tells us the total number of protons plus neutrons at the bottom of each element square on the periodic table.
What is the atomic mass number?
The number of neutrons in this element.
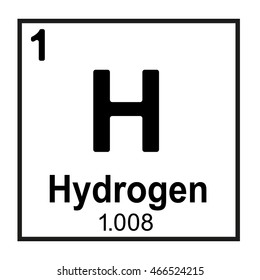
What is zero?
atomic number minus charge if it's positive or the atomic number plus the charge if it's negative
This element has 32 protons and an atomic mass of 95. How many neutrons will it have?
What is 63 neutrons?
Charged atoms are called
ions
What subatomic particle determines the element
protons
How many valence electrons does an element in period 3 group 14 have?
4 Valence electrons
The overall charge of a whole atom is this.
What is zero.
What determines the identity of an element.
What is the number of protons or atomic number?
