What are valence electrons?
Electrons that have the highest energy within an atom.
What is a chemical bond?
The force of attraction that holds atoms together as a result of the rearrangement of electrons between them.
What 3 groups is the periodic table split into?
- metals
- nonmetals
- metalloids
Boron (B)

What is an ion?
An atom or group of atoms that has an electric charge.
True or False?
The electrons of an atom are found at the same energy level.
False.
The electrons of an atom are found at different energy levels.
How many valence electrons allow for an atom to be stable?
The atomic number of an element is the # of _______ in each atom of that element.
Protons
Carbon (C)

True or False?
Ions that are made up of 1 atom is called polyatomic.
False.
Ions that are made up of more than 1 atom are called polyatomic.
**HINT: the prefix poly means "many"
Valence electrons are involved in what kind of bonding?
Chemical
True or False?
When atoms bond, valence electrons can be transferred from one atom to another or shared between atoms.
True!
True or False?
The number of valence electrons decreases from left to right across a period.
False.
The number of valence electrons increases from left to right across a period.
Neon (Ne)

True or False?
In an ionic compound, the total positive charge of all the positive ions is greater than the total negative charge of all the negative ions.
False.
In an ionic compound, the total positive charge of all the positive ions is equal to the total negative charge of all the negative ions.
True or False?
Electrons at higher energy levels have higher amounts of energy.
True!
True or False?
Hydrogen needs only 3 valence electrons to be stable.
False.
Hydrogen needs only 2 valence electrons to be stable.
The elements in the periodic table are in order by increasing __________ __________.
Atomic Number
Phosphorus (P)
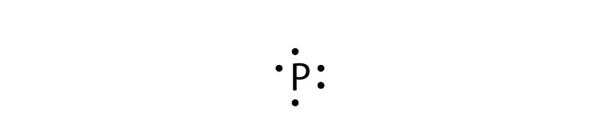
What is an ionic bond?
The attraction between two oppositely charged ions.
The number of valence electrons in each atom helps determine the _________ _________ of that element.
Chemical Properties
Name an example of an atom that has 8 valence electrons.
- Neon (Ne)
- Argon (Ar)
- Krypton (Kr)
- Xenon (Xe)
What would happen if an alkali metal was combined with a halogen?
The alkali metal would give up an electron to the halogen.
Chlorine (Cl)

Your Pick!
When a neutral atom loses a valence electron, it loses a negative charge. It becomes a POSITIVE/NEGATIVE ion.
When a neutral atom gains an electron, it gains a negative charge. It becomes a POSITIVE/NEGATIVE ion.
When a neutral atom loses a valence electron, it loses a negative charge. It becomes a POSITIVE ion.
When a neutral atom gains an electron, it gains a negative charge. It becomes a NEGATIVE ion.