This is the smallest "whole" particle of matter in the universe
An atom!
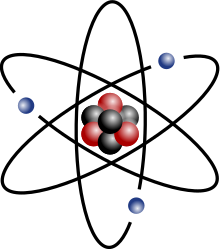
The movement of these particles in an atom is what powers the device you are zooming on right now.
Electrons
This happens when electromagnetic waves hit an object and "bounce" back at the same angle.
Reflection
What are acceptable Units for the Density of your laptop?

g/cm3 or grams/cm3 or kilograms/cm3
True or False: Electrons are attracted to each other and will get as close as possible to other electrons
False!

This type of energy transfer does not require molecules to be touching in order to work. In fact, this type of energy transfer is responsible for all life on the planet Earth!
Radiation
Metals are good _________ of heat and electricity.
Bonus: WHY? hint...
Conductors
Metals conduct heat and electricity well because the electrons of metalic elements move freely from atom to atom in a "sea" of electrons!

What 2 types of energy transfer require molecules to be touching in order to transfer heat?
Conduction and Convection
This is the sum of an atom's protons and neutrons

Atomic Mass
Helium's Atomic Mass = 4-ish
Most models we have to draw of atoms are misleading because...
(2 reasons)

1. The electrons in the drawings are too big! In reality, electrons are 1/1800 the size of protons
2. The electrons in the drawings are too close to the nucleus. In reality, 99% of an atom is empty space!
1. Vocab term for poor conductors of heat and electricity.
2. Each team member find an example and show in their video square!
1. Insulator
2. Plastic, rubber, paper
Describe the type of heat energy transfer happening here...

Bonus: what would happens to the coffee molecules as the spoon molecules get hotter?
Conduction of heat because hot, fast coffee molecules hit the metal spoon molecules and transfer their energy so that the spoon molecules move faster, and hit other spoon molecules and make THEM go faster, all the way to the end of the spoon.
Bonus: Coffee molecules that transfer their heat energy by hitting spoon molecules get "less hotter" and slow down.
Calculate the density of Meg's laptop using the following information...
Mass = 1290 g
Volume = 30 cm wide × 21.2 cm length × 1.6 cm high
Hint: remember UNITS!!!!
Density = Mass/Volume = 1.29/1017.6 =....
1.27 g/cm3
(Volume = 1017.6 cm3)
This is a single atom of what element....

Bonus: How do you know??
Lithium! Because there are 3 protons (red because there are the same number of electrons)
Explain why this circuit is on fire!

There is nothing to provide resistance to the flow of electrons, and thus they produce excessive heat energy. These wires would feel extremely hot to the touch!
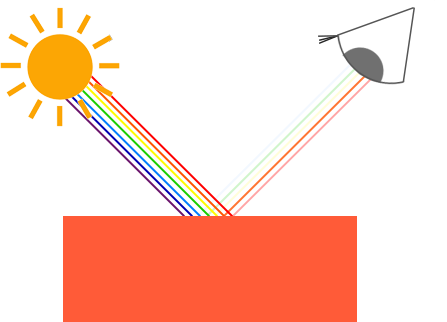
Describe what is happening with the light waves AND ATOMS in this picture...

The green and blue light waves travel at the same frequency as the electron vibrations in the atoms of the object, so when the green and blue light hits the object the light energy is absorbed and transfers into heat energy (aka: the electrons and atoms vibrate more and the temperature of the object increases!). The red light frequency does not match the electrons, so that energy is reflected back out.
(or something like that)
Define the scientific term: Temperature
BONUS: What has faster moving molecules --> A hot, solid metal pan in the oven or a cool glass of liquid water from the fridge?
The measurement of the AVERAGE energy of atoms or molecules in a material or substance.
The solid hot pan!