Is rust an example of a chemical change? Explain
What is YES? You cannot change rust back into the metal is was before; therefore, it is a chemical change.
What type of chemical reaction absorbs heat?
What is endothermic
The chemical formula for table salt.
What is NaCl?
Substance made up of only one kind of atom.
What is an element?
Name the three indicators of a chemical change.
What are color change, bubbles, temperature change, odor, light?
Draw an accurate model representing an Exothermic Reaction.
exothermic reaction is a chemical reaction that releases heat making the surroundings warm.
50 grams of magnesium reacts with oxygen. How many grams of magnesium will be present after the reaction occurs?
What is 50 grams
What is a particle that contains more than one atom joined together?
what is a molecule
Center of the atom that makes up most of the atom's mass.
What is the nucleus?
Name a food that is an example of a chemical change and explain why.
What is a cake, because you cannot turn a cake back into the eggs, flour, sugar, etc. that you mixed together?
According to the Law of Conservation of Mass, if 5 atoms of carbon are used as a reactant, how many atoms of carbon must be a part of the product?
What is 5 atoms
A molecule that has 12 total atoms, consisting of 3 carbon atoms, 1 oxygen atom, and some hydrogen atoms would have this chemical formula.
What is C3H7OH (C3OH8)
Explain how the water cycle an example of a physical change.
What is YES, it is a physical change, because water changes from a liquid/solid when it precipitates, to a gas when it evaporates, then forms back into a liquid when it condensates. Then the process repeats again with the same substance, WATER!
Endothermic or Exothermic? Why?
Exothermic Reaction
(Overall, a loss of energy from the beginning to the end)
What do the products and reactants of a chemical reaction have in common?
What is they have the same amount of matter.
Balance this Equation:

4 Al + 3O2 -> 2AlO3
Draw the Bohr model for neon. (Remember to wear neon on friday!)

Explain how a rocket launch is an example of a chemical change.
What is the fuel combines with another chemcial to produce large volumes of a hot gas that propel the rocket into space?
Endothermic vs Exothermic? Why?
Endothermic Reaction
(Overall, an increase in energy from beginning to end)
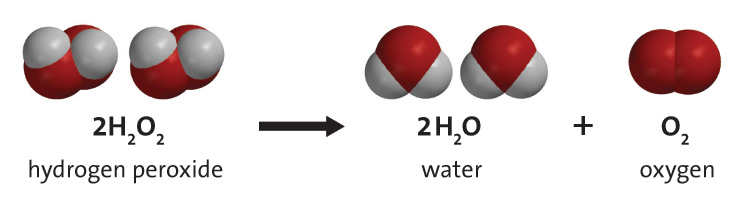 Think back to the liver and hydrogen experiment inside the bag. Explain how the conservation of mass relates to this.
Think back to the liver and hydrogen experiment inside the bag. Explain how the conservation of mass relates to this.
Answers will vary, but should mention the equal amounts on the reactant and product side, the law of conservation of mass, and/or closed system.
Balance the Equation. Remember (You can change coefficients). 
2C2H6 + 7O2 -> 4CO2 + 6H2O

