The smallest unit of an element that has the chemical identity of that element.
What is an atom
A substance only made of one kind of atom that cannot be separated or broken down into simpler substances.
What is an element
True or False- Hydrogen is matter
What is true
The elements on the periodic table each have a unique _________________.
What is number of protons or atomic number?
Record the mass of the object on the triple beam balance below.
What is 538g
This is number tells the number of protons in the nucleus of each atom of an element
What is the atomic number
This type of substances particles can be compressed to fit into a smaller container.
What is gas
This is the positively charged subatomic particle that resides in the nucleus of an atom.
What is a proton?
In addition to being grouped by atomic number, elements on the periodic table are grouped by the __________________ and __________________ that they have in common with other elements.
What are physical and chemical properties
what is the volume of the liquid in the graduated cylinder?
:max_bytes(150000):strip_icc()/meniscus03-58b5b2ea5f9b586046bb3551.png)
What is 38mL
Most of the mass of an atom is located in this part of an atom.
What is the nucleus
Substances in these state(s) of matter have thermal energy
What are solid, liquid and gas
This is a negatively charged subatomic particle in an atom
What is an electron?

How many protons are in Erbium?
What are 68
True or False- heat & light are forms of matter
What is false
This number is the sum of the an atom's protons and neutrons
What is mass number/atomic mass
True/False. If false, make it true.
An example of a chemical change is when water freezes
False, this is a physical change.
Every atom we deal with this year will have an equal number of ___________ and ________________.
What is protons and electrons

Solve for the number of neutrons in Erbium.
What are 99
Solve for the density of the rectangular prism below using the formula d=m/v. Round your response to the nearest tenth.
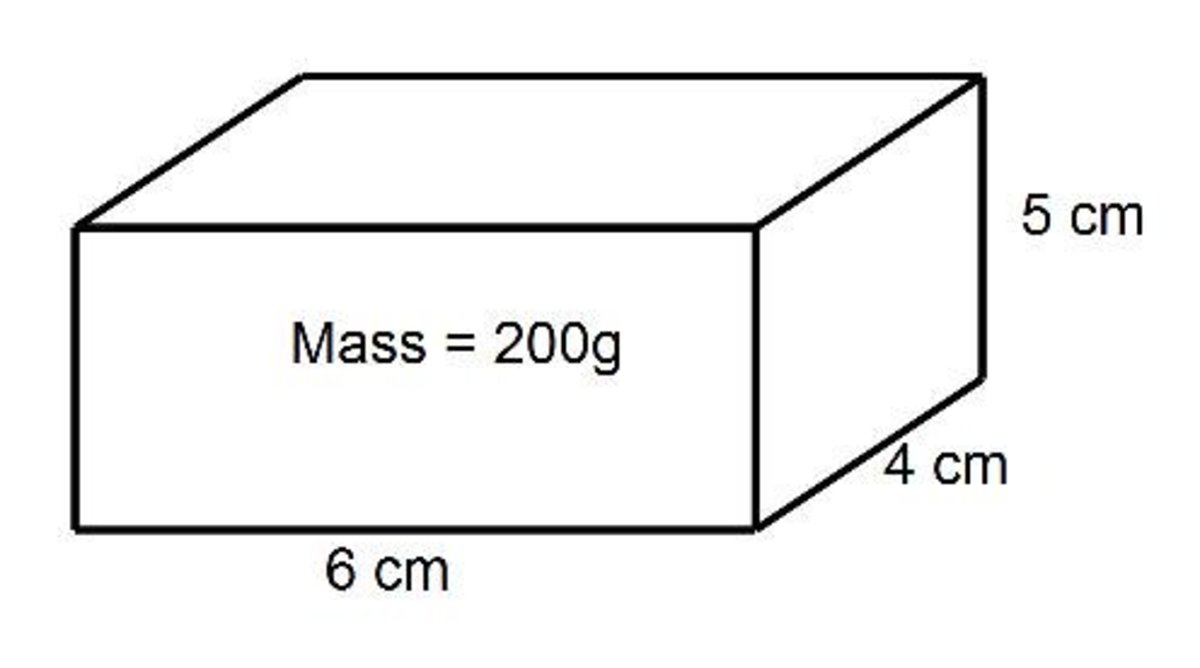 Will the substance sink or float when placed in water?
Will the substance sink or float when placed in water?
What is 1.7g/cm3 and sink
What is the approximate atomic mass of this element?
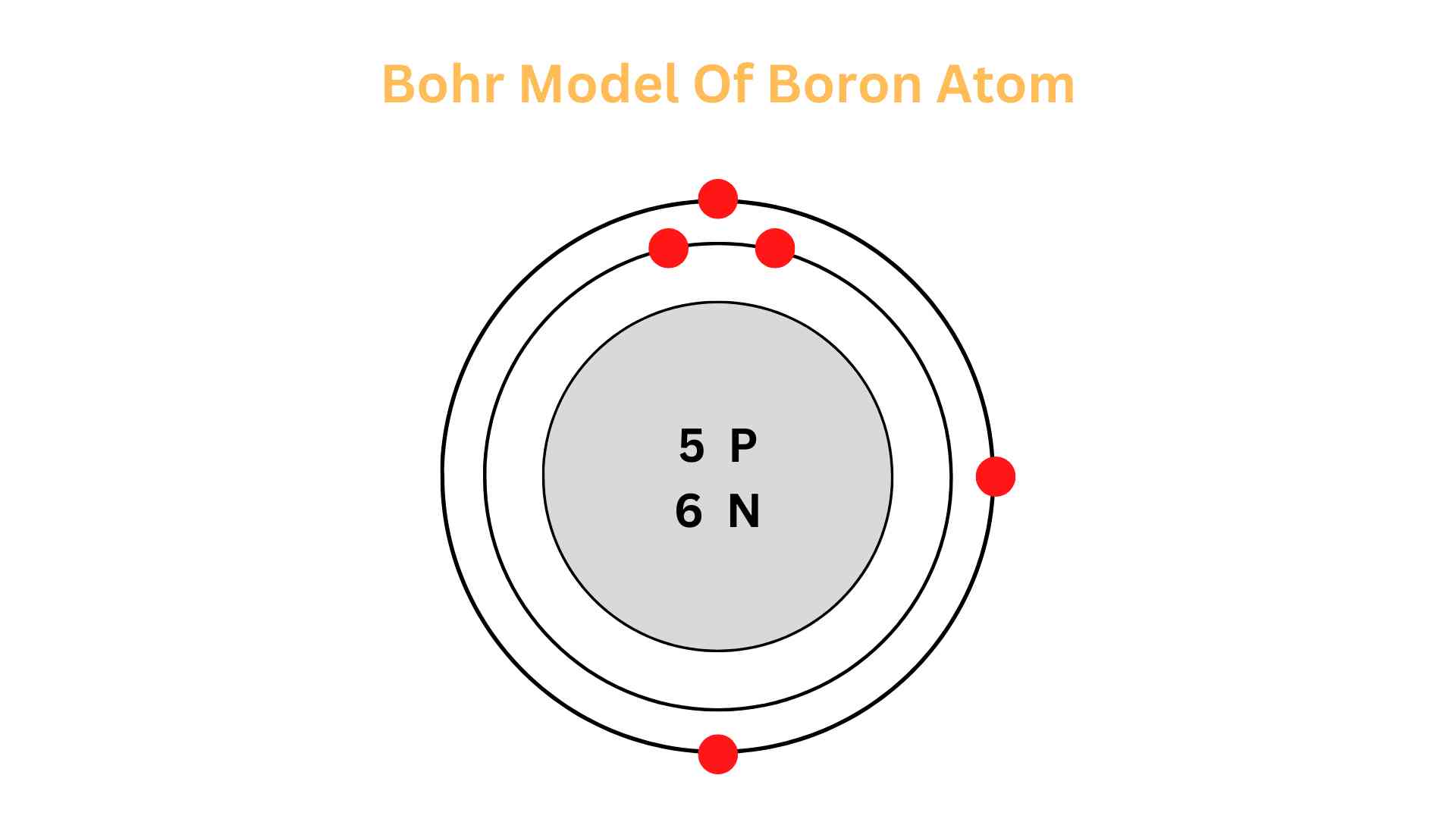
What is about 11amu
A liquid is placed into a freezer. Explain how the arrangement and motion of its particles will change as it changes from a liquid to solid form.
What is the particles will stop "switching positions/flowing" and will begin to vibrate in place
